The Insta NX® Mag 16Plus Automated Nucleic Acid Extractor provides an advanced solution for isolating viral nucleic acids, employing magnetic bead technology to achieve consistent DNA and RNA extraction from a variety of clinical specimens.
Image Credit: RIDVAN_BULBUL/Shutterstock.com
When used with HiPurA® Pre-filled Cartridges/Plates for Viral Nucleic Acid Purification, this system guarantees efficient and high-quality extraction, minimizing reagent waste even for single samples. Validation with NIBSC standards and known viral load samples confirms the method's sensitivity and accuracy, making it suitable for high-sensitivity viral load assessments and diagnostics.
Introduction
Viral diseases pose a significant global health challenge, placing substantial strain on healthcare systems. Accurate identification of viral infections is crucial for monitoring outbreaks, managing chronic diseases, and advancing scientific research.
The effective diagnosis, treatment, and development of vaccines depend on the ability to isolate viral nucleic acids from clinical samples. The success of molecular diagnostics relies heavily on the quality of nucleic acid extraction from various sample types, including blood, serum, plasma, nasopharyngeal or oropharyngeal swabs, and tissue.
Given the specific challenges posed by different viruses—such as variations in viral load, sample inhibitors, and genomic discrepancies, there is a pressing need for efficient, flexible, and dependable extraction methods that yield high-quality results across a wide spectrum of viral infections.
Infections like HIV, HBV, HCV, Dengue, Chikungunya, Influenza A and B, RSV A and B, and SARS-CoV-2 require tailored techniques for effective isolation.
An effective method for one virus might not work well for another, complicating the implementation of a universal approach. Differences in viral loads, the presence of inhibitors in certain specimens, and variations in viral genomes can affect nucleic acid extraction efficiency, highlighting the necessity for adaptable methodologies that guarantee quality results for diverse viral infections.
Enhancing viral diagnostics with Himedia’s automated nucleic acid isolation solution
Emphasizing high-yield, safe, and versatile nucleic acid extraction techniques is vital for accurate diagnostics, viral load analysis, and epidemiological studies. The Insta NX® Mag 16Plus Automated Nucleic Acid Extractor employs magnetic bead technology to ensure reproducible, high-quality isolation of DNA and RNA from a variety of clinical samples.
Capable of processing 1 to 16 samples simultaneously with customizable protocols, it improves both efficiency and accuracy while reducing errors. Coupled with HiPurA® Pre-filled Cartridges/Plates for Viral Nucleic Acid Purification, this system provides a rapid and user-friendly viral nucleic acid purification method, optimizing throughput and resource utilization, particularly for individual samples without waste.
Key features
- Magnetic bead-based isolation
- High throughput
- Automated workflow
- Versatility - multiple sample types
- Consistent and reproducible results
- Scalability
Benefits
- Increased accuracy and reliability
- Greater efficiency
- Faster turnaround time
- Cost-effectiveness
- Standardization of processes
Table 1. Validation of Insta NX® Mag16Plus for detecting different viruses across clinical samples. Source: Himedia Laboratories Private Limited
| Virus detected/ validated |
Sample types validated |
| HIV |
Serum |
| HBV |
Serum, Plasma |
| HCV |
Serum, Plasma |
| Dengue |
Blood, Serum, Plasma |
| Chikungunya |
Blood, Serum, Plasma |
| RSV A and B |
Nasopharyngeal and oropharyngeal swabs |
| SARS-CoV-2 |
Nasopharyngeal and oropharyngeal swabs |
| Influenza A |
Nasopharyngeal and oropharyngeal swabs |
| Influenza B |
Nasopharyngeal and oropharyngeal swabs |
Workflow

Figure 1. Workflow for extraction of Viral Nucleic acid with HiPurA® Pre-filled Plates/Cartridges for Viral Nucleic acid extraction kit on Insta NX® Mag16Plus machine and analysis with real-time PCR. Image Credit: Himedia Laboratories Private Limited
Validation of extraction efficiency and sensitivity using international standards
The extraction range and performance of the viral nucleic acid isolation method were meticulously assessed using National Institute for Biological Standards and Control (NIBSC) standards.
The 5th WHO International Standard for HBV DNA (NIBSC code: 22/120) was utilized to validate the extraction process for HBV, ensuring alignment with international nucleic acid testing (NAT) standards.
This standard was added to negative plasma samples, serially diluted, and extracted in triplicates, followed by the evaluation of a commercial HBV Quantitative Kit. This comprehensive method allowed for a thorough assessment of the extraction's sensitivity, specificity, and reproducibility.
Established reference materials enabled accurate determination of the Limit of Detection (LOD), confirming the efficient isolation of viral nucleic acids from plasma at low concentrations.
Using the NIBSC HBV Standard (22/120), a detection limit as low as 2.39 IU / μL was achieved with HiPurA® Pre-filled Cartridges/Plates for Viral Nucleic Acid Extraction on the Insta NX® Mag 16Plus, as confirmed by real-time PCR results. This affirms the method's suitability for high-sensitivity applications like viral load testing and diagnostic assays, ensuring dependable performance in clinical environments.
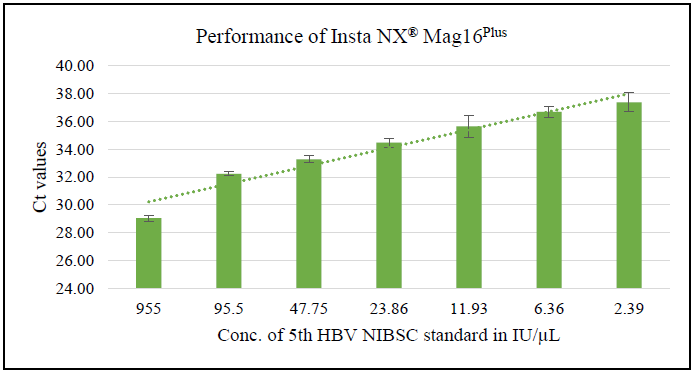
Figure 2. Average Ct for range of input IU/μL of HBV standard spiked in Negative plasma. Image Credit: Himedia Laboratories Private Limited
Validation of extraction efficiency and sensitivity using sample(s) with known viral load (calibrator)
The extraction range and performance of the viral nucleic acid isolation method were tested using a dilution series of known HCV samples.
Plasma samples with an initial HCV viral load of 10 5 IU/ml were serially diluted to concentrations of 105, 104, 103, and 102 IU/ml. These samples were extracted in triplicates and analyzed with a commercial HCV Quantitative Kit.
The method successfully detected HCV down to a dilution of 102 IU/ml, as illustrated in Figure 3. This outcome demonstrates that the nucleic acid extraction method is highly effective and capable of isolating and detecting HCV viral RNA at low concentrations. It confirms the method's suitability for high-sensitivity uses, including viral load testing and diagnostic assays, ensuring reliable detection even in samples with low viral loads.
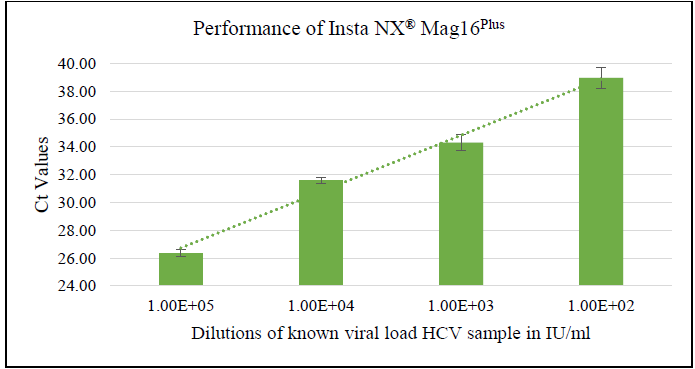
Figure 3. Performance of Insta NX® Mag16Plus kit based on dilution of known quantity of HCV sample. Image Credit: Himedia Laboratories Private Limited
Clinical performance evaluation against other commercial extraction methods
The performance and efficiency of the Insta NX® Mag 16Plus Nucleic Acid Extractor were thoroughly evaluated using clinical samples from patients infected with HCV (n = 50), HBV (n = 40), and HIV (n = 100). These samples were processed with the Insta NX® Mag 16Plus system in conjunction with pre-filled magnetic bead-based extraction kits and compared against commercially available extraction kits. The analysis focused on extraction methods' ability to efficiently and accurately isolate viral nucleic acids.
The results showed a strong concordance between the two extraction methods. All HCV-positive, HBV-positive, and HIV-positive samples tested positive with both the Insta NX® Mag 16Plus and the commercial kit, while negative samples confirmed negative results with both methods, validating the specificity and reliability of the Insta NX® Mag 16Plus system.
These findings demonstrate that the Insta NX® Mag 16Plus can effectively extract viral RNA from various clinical samples, ensuring consistency in diagnostics across different infection types.
Additional analysis of the correlation between Ct values from samples extracted with the commercial kit and Insta NX® Mag 16Plus indicated excellent correlation for HBV and HCV (Figures 4-6). This strong correlation demonstrates that the magnetic bead-based method used by the Insta NX® Mag 16Plus system performs comparably or better, establishing it as a highly effective and reliable option for viral nucleic acid extraction.
These results highlight the efficiency, sensitivity, and reliability of the Insta NX® Mag 16Plus system, positioning it as a cost-effective and scalable solution for clinical diagnostics.
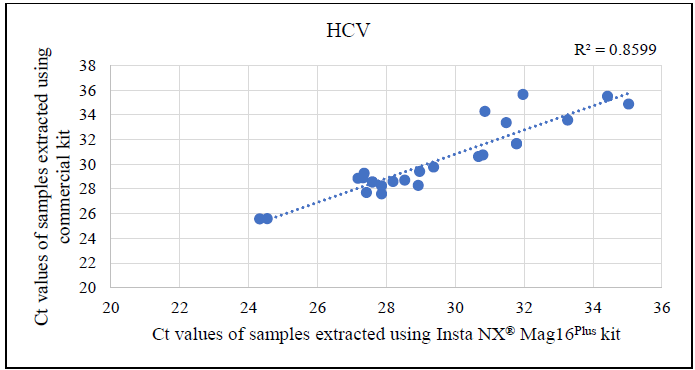
Figure 4. Correlation analysis between cycle threshold (Ct) values derived from HCV real-time PCR using Insta NX® Mag16Plus system compared against commercial kit. Image Credit: Himedia Laboratories Private Limited
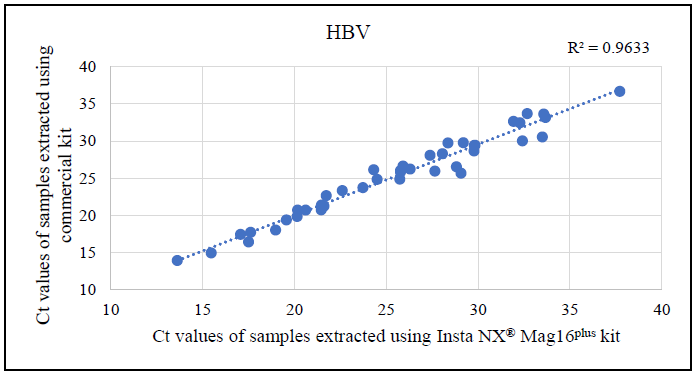
Figure 5. Correlation analysis between cycle threshold (Ct) values derived from HBV real-time PCR using Insta NX® Mag16Plus system compared against commercial kit. Image Credit: Himedia Laboratories Private Limited
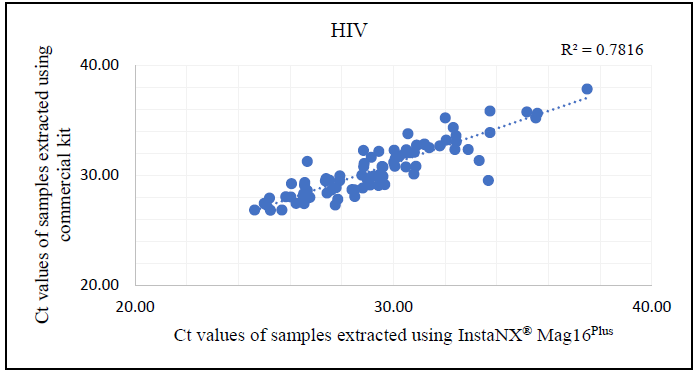
Figure 6. Correlation analysis between cycle threshold (Ct) values derived from HIV real-time PCR using Insta NX® Mag16Plus system compared against commercial kit. Image Credit: Himedia Laboratories Private Limited
Conclusion
The present study illustrates that HiMedia’s Insta NX® Mag 16Plus Nucleic Acid Extractor, together with HiPurA® Pre-filled Cartridges/Plates for Viral Nucleic Acid Extraction, provides a highly sensitive and streamlined approach for extracting nucleic acids from clinical samples infected with viruses.
Integrating pre-filled magnetic bead-based kits significantly improves workflow efficiency, speed, and cost-effectiveness while simplifying the process and reducing labor compared to traditional methods. This combination presents an excellent alternative to existing commercial options, with the potential to enhance accessibility to viral infection diagnostics and contribute to more effective patient identification and treatment monitoring.
About Himedia Laboratories Private Limited
With a presence in more than 150 countries, HiMedia is amongst the top three brands in the Bioscience Industry.
HiMedia Laboratories Private Limited is world renowned for manufacturing high quality culture media for microbiology. Additionally, we provide advanced media and products in the fields of Molecular Biology, Cell Biology, Plant Tissue Culture, Chemicals and Lab Aids/Equipment. As a Top Tier Global player, we are not only dedicated towards products but also striven towards introducing technologies such as Genomics Sequencing Services and Hydroponics.
HiMedia has managed to do this over decades as we have our own in-house bulk raw materials manufacturing plant. This enables us to deliver consistent quality products that conform to ISO 9001:2015 and ISO 13485:2012 and WHO: GMP.
HiMedia Labs. caters to one of the broadest Biosciences product categories: our premier established line of Microbiology products and newer promising products in Molecular Biology, Automated and Molecular Instruments, Cell Biology, Chemicals, and Premium Grade Lab Consumables, amongst others. The COVID-19 pandemic revolutionized not the clinical industry’s thought process regarding the significance of Molecular Diagnostics products.
The ‘Molecular Biology and Virology Division’ of HiMedia Laboratories Pvt. Ltd. Also called as HiGenoMB® is a One Stop Solution Provider churning out potential Research and Industry oriented Molecular biology products for the past glorious decade. About 2000 different products such as Nucleic Acid Extraction and Amplification (PCR) Kits, Cloning Reagents, Buffers & Chemicals for proteomics studies, Automated Molecular Instrumentation including RT PCR machines and PCR thermal cyclers and DNA/RNA Extraction platforms are being produced. The Proficient researchers in this department are spear heading the challenging field of Molecular Diagnostics to provide a complete solution for clinical diagnosis, agriculture, veterinary sciences, food industry, drug discovery and forensic medicine with the use of Real Time PCR or quantitative PCR kits and thermal cyclers. Our Molecular Biology Division-has established an in-house Advanced Sequencing and Bioinformatics facility which marks HiMedia’s entry into the Services space.
Our Cell Biology segment contributes with technologies which have brought in Serum free media for biopharma applications, Viral Vaccine Production Platform, Multicompendial grade chemicals, cultivated meat, and 3D bioprinting.
Moving from conventional to advanced automated methods like MALDI-TOF (Autof MS 1000) has been our newest endeavour for Microbiology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.