Dengue virus (DENV) continues to pose a substantial global health risk, with four distinct serotypes (DEN-1, DEN-2, DEN-3, DEN-4) circulating in areas where Aedes mosquitoes thrive. Accurate serotyping of DENV is vital for understanding outbreak epidemiology, managing patient treatment, and implementing vector control measures.
Image Credit: Kateryna Kon/Shutterstock.com
The Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 provides a quick, sensitive, and dependable method for the simultaneous detection and differentiation of all four dengue serotypes. Utilizing real-time PCR technology with specialized hydrolysis probes, the kit generates results within 90 minutes, making it an essential tool for clinical diagnostics, surveillance, and research.
Introduction
Dengue fever, caused by the Dengue virus (DENV), represents a major vector-borne infectious disease with worldwide implications. The presence of four distinct DENV serotypes (DEN-1, DEN-2, DEN-3, and DEN-4) means that infection with one serotype does not provide immunity against the others, increasing the risk of severe illness during subsequent infections with a different serotype.
The complexity of the disease and the potential for severe outcomes, such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), highlight the necessity for accurate diagnosis and effective serotyping.
Historically, diagnosing and distinguishing DENV serotypes has been complicated due to the similarities in clinical presentation and the requirement for specialized testing. However, the emergence of multiplex PCR technology has facilitated rapid and accurate detection and serotyping of DENV in clinical samples.
The Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 is specifically designed to address this need, providing a streamlined and reliable solution for clinicians and epidemiologists to swiftly and accurately diagnose and differentiate DENV serotypes.
The science behind Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 - Overview of the technology
The Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 employs real-time PCR technology to amplify viral RNA from clinical samples, followed by detection with fluorescent probes. This approach allows for the swift and precise differentiation of all four dengue serotypes (DEN-1, DEN-2, DEN-3, and DEN-4) within a single assay. The kit is designed to enhance viral RNA amplification through reverse transcription, enabling detection of even low viral loads.
At the heart of this kit are hydrolysis probes, which are short oligonucleotides featuring a fluorescent reporter dye at the 5' end and a quencher dye at the 3' end. These probes exhibit high specificity for each of the four DENV serotypes, facilitating precise identification during the amplification process. When the target RNA is detected, the probe attaches to the target sequence and is cleaved by the polymerase enzyme, thereby emitting a fluorescent signal for detection. Such real-time monitoring enables prompt data collection and analysis.
The Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 comprises two separate tubes: Tube 1 contains primers and probes for DENV-2, DENV-3, and DENV-4, each labeled with different fluorophores (FAM, Texas Red, and Cy5). Tube 2 includes primers and probes for DENV-1, labeled with the JOE fluorophore, along with an internal control amplification system to ensure the accuracy of RNA isolation and PCR efficiency. This two-tube format facilitates clear differentiation among the four serotypes while verifying RNA integrity.
Benefits and applications
Molecular features
- Simultaneous serotyping of Dengue viruses (serotypes 1-4) in a single assay
- High sensitivity – 1-2 copies for the DENV 1-4 serotypes
- High specificity – No cross-reactivity with other common flavivirus and arbovirus family
Technology features
- Rapid and reliable results within 90 minutes
- One-step assay (i.e. the same tube can perform reverse transcription and amplification)
- Includes all reagents & controls for test validation
- Open system – Compatible with 4-channel and 5-channel qPCR cyclers
- Wet-lab assays validated on the Bio-Rad CFX Opus 96, Applied Biosystems QuantStudio 5 and Insta Q96® Plus Real Time PCR Systems
Applications
- Clinical diagnostics
- Epidemiological surveillance
- Outbreak monitoring
- Vaccine development and research
Workflow
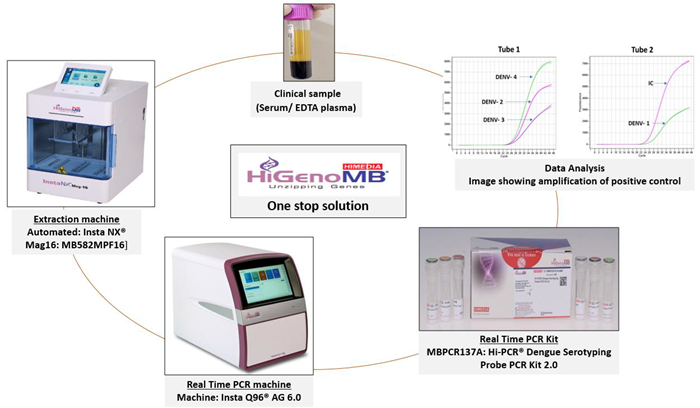
Figure 1. Image representing probe workflow of the process and One Stop Solution offered by HiGenoMB. Image Credit: Himedia Laboratories Private Limited
Performance validation
The Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 has undergone thorough validation across multiple platforms, including Bio-Rad CFX Opus 96, Applied Biosystems QuantStudio 5, and Insta Q96® Plus Real-Time PCR Systems.
Analytical sensitivity
Limit of Detection (LoD)
The Limit of Detection (LoD) is defined as the concentration (copies per μl of the eluate) of the target molecule that can be detected with a 95% probability, in accordance with CLSI EP17-A2.
The LoD assay for the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 was conducted using 20 replicates on Biorad CFX Opus 96, Applied Biosystems QuantStudio 5, and Insta Q96® Plus systems with synthetic nucleic acid for dengue serotypes DENV-1, DENV-2, DENV-3, and DENV-4. The detectable limits were found to be 1.4 copies per μL for DENV-1, 1 copy per μL for DENV-2, 1.9 copies per μL for DENV-3, and 1.4 copies per μL for DENV-4.
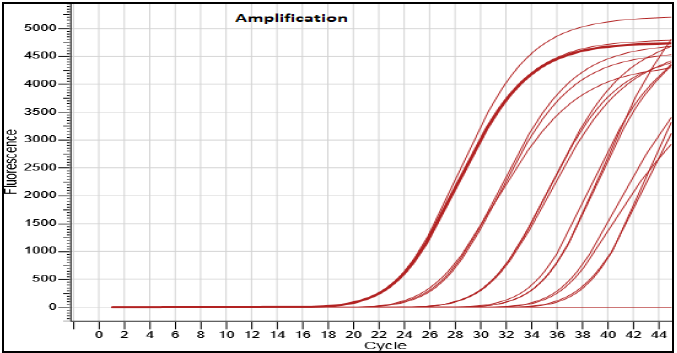
Figure 2. Dilution series (5622 copies/μl, 562 copies/μl, 56.2/μl copies, 5.62/μl copies and 0.56/μl) of Dengue Serotype 2 nucleic acid template run on the Insta Q96® Plus Real Time PCR Systems. Image Credit: Himedia Laboratories Private Limited
Analytical specificity
Inclusivity – In silico
The analytical specificity of the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 was ensured through in-silico analysis of the oligonucleotides (primers and probes). The sequences of all targets were compared against relevant DENV sequences available in the GenBank database.
Analytical reactivity
The analytical reactivity of the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 was confirmed through wet lab testing of the oligonucleotides against commercially available controls, including AMPLIRUN® DENGUE VIRUS RNA CONTROLS from Vircell Microbiologics [DENV 1 (Hawaii strain), DENV 2 (New Guinea C strain), DENV3 (H87 strain) and DENV4 (H241 strain) and BEI Resources [Dengue virus type 1 (DEN-1), Hawaii, Dengue virus type 2 (DEN-2), DENV-2/US/BID-V594/2006, Dengue virus type 3 (DEN-3), DENV-3/US/BIDV1043/2006 and Dengue virus type 4 (DEN-4), UIS 497].
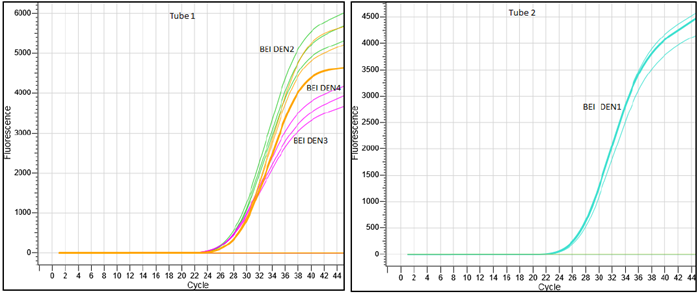
Figure 3. Image representing probe based Real-Time amplification plots of BEI Resources DENV 1-4 serotypes confirmed by Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 on Insta Q96® Plus Real Time PCR System. Image Credit: Himedia Laboratories Private Limited
Cross-reactivity and interference with other microorganisms
Wet testing was performed against genomic or synthetic DNA/RNA from various pathogens (from ATCC) using Bio-Rad CFX Opus 96 and Insta Q96® Plus systems to assess potential cross-reactivity. None of the pathogens tested showed reactivity to the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 primers and probes.
Table 1. List of organisms tested by wet lab assays for cross reactivity determination. Source: Himedia Laboratories Private Limited
| |
|
| Human coronavirus 229E (VR-740D) |
Plasmodium falciparum strain 3D7 (PRA-405) |
Influenza A virus H1N1 strain A/California/07/2009
(H1N1) pdm09 (VR-1894DQ) |
Plasmodium vivax DNA (PRA-3004SD) |
| Influenza A virus (H3N2) strain A/Wisconsin/15/2009 (VR-1882DQ) |
Leptospira interrogans serovar Copenhageni strain Fiocruz
L1-130 (BAA-1198D-5) |
| Influenza B virus strain B/Florida/4/2006 (VR-1804DQ) |
Staphylococcus aureus subsp. aureus (43300DQ) |
| Hepatitis B virus (VR-3232SD) |
Human respiratory syncytial virus strain 18537 (VR-1580DQ) |
| Hepatitis C virus (VR-3233SD) |
Human herpesvirus 1 MacIntyre (VR-539DQ) |
| Hepatitis A virus (VR-3257SD) |
Human herpesvirus 2 (VR-540DQ) |
| Hepatitis E virus (VR-3258SD) |
Human metapneumovirus (VR-3250SD) |
| Human immunodeficiency virus 1 (HIV-1) RNA (VR-3245SD) |
Candida albicans strain SC5314 (MYA-2876DQ) |
| Middle East respiratory syndrome coronavirus (VR-3248SD) |
Mycobacterium tuberculosis strain H37Ra (25177DQ) |
| Human parainfluenza virus 1 strain C35 (VR-94DQ) |
Chlamydophila pneumoniae strain CM-1 (VR-1360DQ) |
| Human parainfluenza virus 2 strain Greer (VR-92DQ) |
Streptococcus pyogenes strain Bruno (19615DQ) |
| Human parainfluenzavirus 3 strain C 243 (VR-93DQ) |
Mycoplasma pneumoniae strain M129-B7 (29342DQ) |
| Zika virus (VR-3252SD) |
Legionella pneumophila subsp. pneumophila (33152DQ) |
| Chikungunya virus (VR-3246SD) |
Haemophilus influenzae (51907DQ) |
| Enterovirus 68 strain Fermon (VR-1826DQ) |
Human rhinovirus 16 strain 11757 (VR-283DQ) |
| Human adenovirus 1 strain Adenoid 71 (VR-1DQ) |
|
Cross-reactivity analysis – In silico
The oligonucleotide sequences in the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 underwent BLAST analysis against organisms from the Flavivirus family, those involved in viral hemorrhagic fevers, mosquito-borne diseases, or acute febrile illnesses. No significant cross-reactivity was noted for all evaluated sequences.
Table 2. List of organisms tested by in-silico analysis for cross reactivity determination. Source: Himedia Laboratories Private Limited
| |
|
| West Nile virus (taxid:11082) |
Brucella melitensis (taxid:29459) |
| Yellow fever virus (taxid:11089) |
Borrelia burgdorferi (taxid:139) |
| Yellow fever virus group (taxid:40005) |
Orientia tsutsugamushi (taxid:784) |
| Japanese encephalitis virus (taxid:11072) |
Salmonella (taxid:590) |
| Japanese encephalitis virus group (taxid:11071) |
Corynebacterium diphtheriae (taxid:1717) |
| Spondweni virus (taxid:64318) |
Burkholderia sp. (taxid:36773) |
| Spondweni virus group (taxid:297696) |
Leptospira (taxid:171) |
| Tick-borne encephalitis virus (taxid:11084) |
Plasmodium (taxid:5820) |
| Tick-borne encephalitis virus group (taxid:29263) |
Hepatitis B virus (taxid:10407) |
| Zika virus (taxid: 64320) |
Hepatitis C virus (taxid:11103) |
| Chikungunya virus (taxid: 37124) |
Hepatitis E virus (taxid:291484) |
| Modoc virus (taxid:64300) |
Human hepatitis A virus (taxid:208726) |
| Tamana bat virus (taxid:161675) |
Human immunodeficiency virus (taxid:11676) |
| Cell fusing agent virus (taxid:31658) |
Cytomegalovirus (taxid:10358) |
| Omsk hemorrhagic fever virus (taxid:12542) |
Epstein-Barr virus (taxid:10376) |
| St. Louis encephalitis virus (taxid:11080) |
Human adenovirus sp. (taxid:1907210) |
| Murray Valley encephalitis virus (taxid:11079) |
Human parvovirus B19 (taxid:10798) |
| Kyasanur Forest disease virus (taxid:33743) |
Monkeypox virus (taxid:10244) |
| Rift Valley fever virus (taxid:11588) |
Wesselsbron virus (taxid:164416) |
Israel turkey
meningoencephalomyelitis virus (taxid:64291) |
Measles morbillivirus (taxid:11234) |
| Ntaya virus (taxid:64292) |
Mumps orthorubulavirus (taxid:2560602) |
| Tembusu virus (taxid:64293) |
Rubella virus (taxid:11041) |
| Sepik virus (taxid:44026) |
Hantavirus (taxid:1980442) |
| Usutu virus (taxid:64286) |
Enterovirus (taxid:12059) |
| Aroa virus (taxid:64303) |
Ebola virus (taxid:1570291) |
| Bussuquara virus (taxid:64304) |
Varicella Zoster Virus (taxid:10335) |
| Iguape virus (taxid:64308) |
agent of lymphatic filariasis (taxid:6279) |
| Naranjal virus (taxid:64313) |
Coxiella burnetii (taxid:777) |
| Cacipacore virus (taxid:64305) |
Staphylococcus aureus (taxid:1280) |
| Koutango virus (taxid:44025) |
Streptococcus pneumoniae (taxid:1313) |
| Alfuy virus (taxid:44017) |
Pseudomonas aeruginosa (taxid:287) |
| Kunjin virus (taxid:11077) |
Streptococcus pyogenes (taxid:1314) |
| Yaounde virus (taxid:64319) |
Burkholderia pseudomallei (taxid:28450) |
| Kokobera virus (taxid:44024) |
Leishmania donovani (taxid:5661) |
| Stratford virus (taxid:44027) |
Listeria monocytogenes (taxid:1639) |
| Bagaza virus (taxid:64290) |
Mycobacterium tuberculosis (taxid:1773) |
| Ilheus virus (taxid:59563) |
Klebsiella pneumoniae (taxid:573) |
| Rocio virus (taxid:64315) |
Rickettsia sp. (taxid:789) |
Lymphocytic choriomeningitis
virus (taxid:3052303) |
Bacillus anthracis (taxid:1392) |
| Salmonella paratyphi (taxid:54388) |
Neisseria meningitidis (taxid:487) |
| Aspergillus (taxid:5052) |
Yersinia pestis (taxid:632) |
| Histoplasma (taxid:5036) |
Acinetobacter baumannii (taxid:470) |
Precision studies
The precision of the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 was assessed under various conditions: intra-assay variability (within a single experiment), inter-assay variability (between different experiments), inter-day variability (across three different days), inter-operator variability (among three different operators), inter-instrument variability (between three different PCR thermal cyclers), and inter-lot variability (among three different lots of the kit).
Variability data were evaluated based on standard deviation (SD) and coefficient of variation (CV) derived from threshold cycle (Ct) values. Total variability was determined by combining results from all five types of analyses.
Table 3. Data showing variability observed from Precision (repeatability and reproducibility) studies. Source: Himedia Laboratories Private Limited
| Repeatability and Reproducibility data |
| |
Variability |
DENV-2 |
DENV-3 |
DENV-4 |
DENV-1 |
IC |
| Mean (Ct) |
Intra-assay |
28.183 |
27.434 |
27.746 |
27.041 |
26.253 |
| SD |
0.125 |
0.149 |
0.161 |
0.073 |
0.135 |
| %CV |
0.444 |
0.543 |
0.579 |
0.268 |
0.515 |
| Mean (Ct) |
Inter-assay |
28.168 |
27.530 |
27.853 |
27.052 |
26.294 |
| SD |
0.022 |
0.136 |
0.152 |
0.015 |
0.057 |
| %CV |
0.078 |
0.496 |
0.546 |
0.055 |
0.218 |
| Mean (Ct) |
Inter-Day |
28.193 |
27.317 |
28.143 |
26.767 |
28.884 |
| SD |
0.416 |
0.381 |
0.403 |
0.463 |
0.572 |
| %CV |
1.475 |
1.394 |
1.433 |
1.728 |
1.980 |
| Mean (Ct) |
Inter-Operator |
27.970 |
27.146 |
27.953 |
26.272 |
28.578 |
| SD |
0.115 |
0.084 |
0.131 |
0.195 |
0.051 |
| %CV |
0.412 |
0.308 |
0.467 |
0.743 |
0.179 |
| Mean (Ct) |
Inter-Instrument |
27.655 |
27.211 |
28.329 |
26.738 |
28.937 |
| SD |
0.466 |
1.243 |
1.011 |
0.548 |
0.414 |
| %CV |
1.686 |
4.567 |
3.567 |
2.049 |
1.429 |
| Mean (Ct) |
Inter-lot |
28.149 |
27.532 |
27.969 |
26.667 |
29.237 |
| SD |
0.163 |
0.181 |
0.198 |
0.079 |
0.096 |
| %CV |
0.029 |
0.214 |
0.046 |
0.006 |
0.010 |
| Mean (Ct) |
Total variability |
28.053 |
27.361 |
27.998 |
26.756 |
28.909 |
| SD |
0.193 |
0.149 |
0.191 |
0.262 |
0.234 |
| %CV |
0.689 |
0.545 |
0.680 |
0.978 |
0.809 |
Clinical performance evaluation
The performance of the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 was evaluated using clinical specimens to confirm various aspects of assay performance. A total of 197 clinical samples were analyzed and compared with a Reference CE-IVD PCR Multiplex Kit.
Viral nucleic acids were extracted using the CE-IVD-approved HiPurA® Pre-filled Plates for Viral Nucleic Acid Purification (Cat no: MB582MPF96). Results are shown in the table below.
Table 4. Table representing Sensitivity, Specificity, PPV and NPV data of the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0. Source: Himedia Laboratories Private Limited
| |
Value |
95% CI |
| Diagnostic sensitivity |
100% |
95.01% - 100% |
| Diagnostic specificity |
97.14% |
91.27% – 99.26% |
| Positive Predictive Value |
96.84% |
90.389% - 99.18% |
| Negative Predictive Value |
100% |
95.48% – 100% |
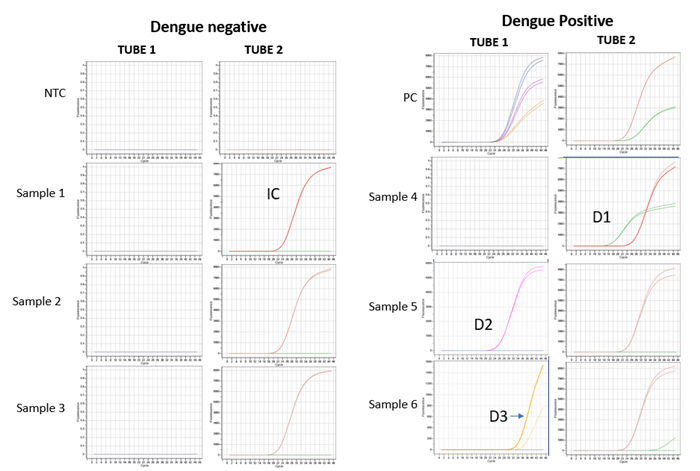
Figure 4. Image representing probe based Real-Time amplification plots of different DENV serotypes and IC detected in clinical samples using Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 on Insta Q96® Plus Real Time PCR System. Image Credit: Himedia Laboratories Private Limited
Case study
RNA extracted from 18 dengue-positive samples was serially diluted at 1:10 or 1:100 ratios to simulate low-titer conditions. These samples were tested with the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 and compared to the Reference CE-IVD PCR Multiplex Kit.
The findings demonstrated excellent concordance between both methods, with one exception: two samples were detected as positive by the Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 (albeit with late Ct values), while the Reference CE-IVD PCR Multiplex Kit reported late Ct values below the detection cutoff, resulting in negative results.
Table 5. Table showing comparison of data of two low titre samples evaluated by Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 and Reference CE-IVD PCR Multiplex Kit. Source: Himedia Laboratories Private Limited
| |
Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 |
Reference CE-IVD PCR Multiplex Kit |
| LoD as per IFU |
DENV1 -2.87 copies/ul; DENV2 <1 copy/ul;
DENV3 -1.87 copies/ul; DENV4 -1.41 copies/ul
[Ct cut-off ≤ 40] |
DENV1-4 – 1 copy/ul for all serotypes
[Ct cut-off ≤ 40] |
| Diluted dengue positive samples tested to mimic low titre samples |
| S28 |
DENV2 detected |
Negative [Ct cut-off ≤ 40] |
| CT: 37.85 |
CT: 40.23 |
| S29 |
DENV4 detected |
Negative [Ct cut-off ≤ 40] |
| CT: 37.89 |
CT: 41.82 |
Conclusion
The Hi-PCR® Dengue Serotyping Probe PCR Kit 2.0 is a robust tool for the rapid and accurate detection and differentiation of all four dengue serotypes. Its high sensitivity, specificity, and user-friendly design make it an indispensable resource for clinicians, public health officials, and researchers combating dengue.
This kit plays a vital role in managing outbreaks, improving clinical outcomes, and supporting efforts to control and prevent dengue worldwide by facilitating timely diagnoses and informed decision-making.
About Himedia Laboratories Private Limited
With a presence in more than 150 countries, HiMedia is amongst the top three brands in the Bioscience Industry.
HiMedia Laboratories Private Limited is world renowned for manufacturing high quality culture media for microbiology. Additionally, we provide advanced media and products in the fields of Molecular Biology, Cell Biology, Plant Tissue Culture, Chemicals and Lab Aids/Equipment. As a Top Tier Global player, we are not only dedicated towards products but also striven towards introducing technologies such as Genomics Sequencing Services and Hydroponics.
HiMedia has managed to do this over decades as we have our own in-house bulk raw materials manufacturing plant. This enables us to deliver consistent quality products that conform to ISO 9001:2015 and ISO 13485:2012 and WHO: GMP.
HiMedia Labs. caters to one of the broadest Biosciences product categories: our premier established line of Microbiology products and newer promising products in Molecular Biology, Automated and Molecular Instruments, Cell Biology, Chemicals, and Premium Grade Lab Consumables, amongst others. The COVID-19 pandemic revolutionized not the clinical industry’s thought process regarding the significance of Molecular Diagnostics products.
The ‘Molecular Biology and Virology Division’ of HiMedia Laboratories Pvt. Ltd. Also called as HiGenoMB® is a One Stop Solution Provider churning out potential Research and Industry oriented Molecular biology products for the past glorious decade. About 2000 different products such as Nucleic Acid Extraction and Amplification (PCR) Kits, Cloning Reagents, Buffers & Chemicals for proteomics studies, Automated Molecular Instrumentation including RT PCR machines and PCR thermal cyclers and DNA/RNA Extraction platforms are being produced. The Proficient researchers in this department are spear heading the challenging field of Molecular Diagnostics to provide a complete solution for clinical diagnosis, agriculture, veterinary sciences, food industry, drug discovery and forensic medicine with the use of Real Time PCR or quantitative PCR kits and thermal cyclers. Our Molecular Biology Division-has established an in-house Advanced Sequencing and Bioinformatics facility which marks HiMedia’s entry into the Services space.
Our Cell Biology segment contributes with technologies which have brought in Serum free media for biopharma applications, Viral Vaccine Production Platform, Multicompendial grade chemicals, cultivated meat, and 3D bioprinting.
Moving from conventional to advanced automated methods like MALDI-TOF (Autof MS 1000) has been our newest endeavour for Microbiology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.