When therapeutic proteins form aggregates, they can limit clinical effectiveness and possibly provoke a serious immunogenic response. Thermostability screening by way of a temperature ramp is a frequently used approach to evaluate the aggregation propensity of various candidate molecules and formulation conditions.
This type of screening is typically conducted in a high-throughput manner with the application of dynamic light scattering (DLS), intrinsic fluorescence and/or other methods to quantify critical attributes such as the melting temperature (Tm) or the onset temperature of aggregation (Tagg).

The DynaPro NanoStar utilizes an intuitive on-board app, DYNAMICS Touch, for guided measurements and walk-up operation. Advanced analyses such as onset temperature are carried out using the full-fea-tures DYNAMICS software, which runs on a standard Windows PC. Image Credit: Wyatt Technology
Accurate determination of Tagg is crucial for the risk assessment of biotherapeutic products. As multi-domain proteins, monoclonal antibodies are exposed to many unfolding steps and resultingly various aggregation kinetics throughout thermal screening.
This article outlines the effect of thermal ramp rate on the unfolding and aggregation behavior of trastuzumab (Herceptin®). A DynaPro® NanoStar® cuvette-based DLS/SLS instrument illustrates that the choice of heating rate throughout thermostability screening is a key factor when acquiring meaningful results.
Introduction
Monoclonal antibodies (mAbs) are among the most frequently exploited class of biotherapeutics due to their capacity to directly target cells and tissue for treatment or precision delivery of small molecular drugs. Circumstances in which mAb-based drugs are used include cancer, infectious diseases, asthma and cardiovascular diseases.
Since mAbs are generally comprised of two light chains and two heavy chains, arranged into three domains, with variations in structure and stability, their unfolding typically leads to a number of intermediate states that aggregate with different possibilities.
The impact of mAb aggregation in pharmaceutical products ranges from simply a loss in efficacy to severe immunogenicity in patients. Therefore, accurate characterization of antibody stability and precision has become a critical requirement for effective screening and approval of mAb-based therapeutics.1
The importance of ramp rate
Thermostability screening is a technique that has the ability to assess physiochemical properties of a protein over a temperature ramp. This kind of experiment typically includes screening various formulation conditions such as pH values, salt concentrations and excipients.
Using a DynaPro Plate Reader, experiments can be automated in conventional microwell plates for high throughput analysis.
While simple proteins thermostability experiments may not be influenced by the heating rate, there are exceptions like mAbs - with their multistep unfolding - where selecting the appropriate heating speed matters.
Heating rates that are deemed excessive may result in relatively slow unfolding or aggregation events assigned to a higher temperature onset while occurring at lower temperatures.
This occurrence may result in assuming higher stability than is actually the case. Therefore, it is not only good practice but of absolute necessity to compare results acquired at the same experimental settings, and to evaluate the influence of temperature ramp rate on the measured Tagg.
DLS is an excellent technique that establishes hydrodynamic radius (Rh), a parameter that increases in relation to the native state with both unfolding and aggregation. Pairing DLS with temperature ramps facilitates the quantification of Tm or Tagg by measuring changes in size across the temperature range.
If static light scattering (SLS) is recorded synchronously, unfolding may be set apart from aggregation by evaluating changes in the weight-average molar mass (Mw) for the analyte.
Measuring DLS and SLS across a temperature ramp
The DynaPro NanoStar simultaneously measures DLS and SLS using dedicated, optimized detector modules for every kind of measurement. It also offers exceptional temperature control, which offsets the lag in solution temperature in relation to instrument temperature that occurs as a result of the thermal mass of the quartz cuvette and liquid.
This generates an accurate ramp across an extensive temperature range. Just 2 µL of sample can be analyzed for each candidate or condition.
The NanoStar was used to assess the impact of ramp rate on the apparent thermostability of trastuzumab (Herceptin), a mAb that is commercially available and used in the treatment of breast cancer.
The study investigated the impact of heating rate on the measured value of Tonset, the temperature at which unfolding or aggregation generates an increase of Rh and Mw.
Materials and methods
Trastuzumab was acquired in powder form (Sigma-Aldrich) and resolubilized in a conventional PBS buffer to a final concentration of 1 mg/mL and then filtered through a 0.02 µm syringe-tip filter.
For each of the thermal ramp experiments, 2 µL of the trastuzumab solution was passed into a clean, calibrated quartz cuvette and a drop of silicon oil was carefully positioned top to impede evaporation. Thermostability was then evaluated using DLS and SLS in a DynaPro NanoStar.
Both Rh and Mw were measured across a temperature range that varied from 25 °C to 90 °C with ramp rates from 0.1 °C/minute to 2.0 °C/minute. Data acquisition was completed at each temperature point over 3 acquisitions of 3 seconds each.
Cumulant Onset analyses were conducted using DYNAMICS® software to calculate Rh and Tonset. Distribution plots were produced using the NNLS regularization analysis as a basis.
Results and discussion
DLS analysis shows that Rh of 5.5 nm for trastuzumab at 25 °C, which is common for a monomeric, natively folded mAb. 2 Mw of ~140 kDa measured by SLS highlights the fact that the mAb is monomeric as it is similar to the molar mass calculated from sequence of ~150 kDa.
Figure 1a demonstrates the regularization histogram with a single narrow peak, indicating the presence of only monomeric antibodies at 25 °C and therefore, the samples are initially free of higher-order species or aggregates.
Figure 1b demonstrates how thermal stress results in aggregation, presented by the regularization histogram at 90 °C.
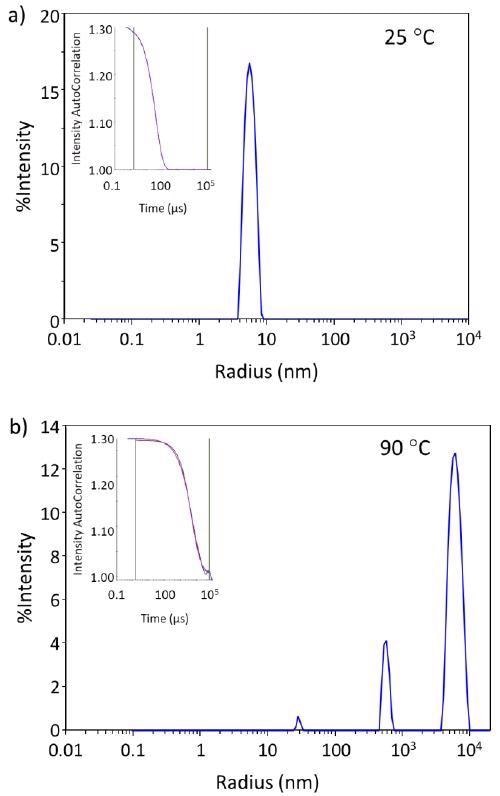
Figure 1. The autocorrelation functions and regularization histograms of trastuzumab at 25 °C and 90 °C. Image Credit: Wyatt Technology
Thermostability in slow and fast ramps
The impact of the heating rate on the so-called thermostability of trastuzumab was carefully assessed by recording DLS and SLS data while conducting temperature ramps at various rates.
Table 1 shows the Tonset for each heating rate and measurement type, while Figure 2 highlights a comparison of the thermostability measured during the slowest heating rate (0.1 °C/minute) and the fastest heating rate (2.0 °C/minute) as applied throughout this study.
Table 1. Trastuzumab temperature of onset (Tonset) calculated from DLS (Rh) or SLS (Mw) recorded during different heating rates. Source: Wyatt Technology
Heating Rate
(°C/minute) |
Tonset from DLS
(°C) |
Tonset from SLS
(°C) |
| 0.10 |
71.8 |
71.0 |
| 0.25 |
74.3 |
73.5 |
| 0.50 |
75.6 |
74.8 |
| 1.00 |
77.2 |
76.8 |
| 2.00 |
78.2 |
77.8 |
Both Rh and Mw of the mAb seem to be unaffected up to 70 °C (Figure 2). Above this threshold, there is an increase in both radius and molar mass.
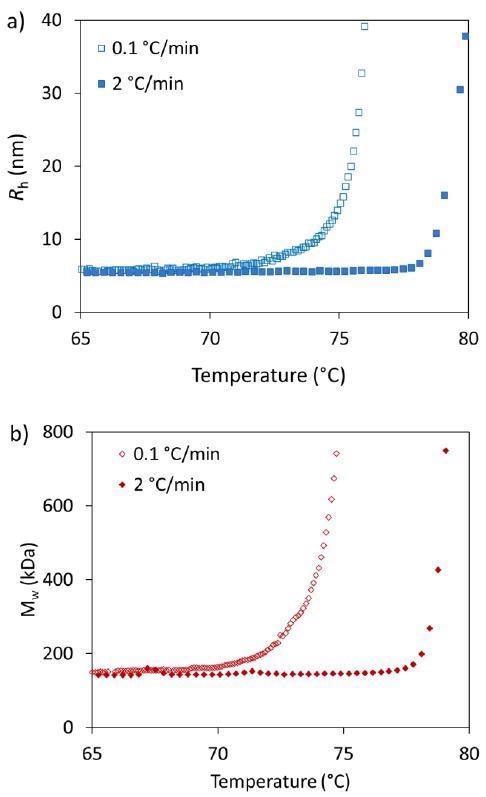
Figure 2. Influence of heating rates on the thermostability characterization of trastuzumab by a) DLS (Rh) and b) SLS (Mw). Image Credit: Wyatt Technology
Tonset from DLS is 71.8 °C for the slowest and 78.2 °C for the most rapid heating rate. A similar result is acquired when calculating Tonset from SLS: 71.0 °C and 77.8 °C for slowest and fastest heating rate, respectively.
An increase in Rh and Mw occurs at around the same onset, which demonstrates that aggregation is actively occurring rather than just unfolding. The mAb seems to aggregate at lower temperatures if the heating rate is slower.
This reveals that early unfolding takes place around ~71 °C, which in turn results in slow aggregation.
The combination of slow aggregation at 71 °C and the rapid transition in temperature for the high ramp rate generates an apparent increase of 7 °C in Tonset at 2.0 °C/minute. Therefore, it is obvious that the choice of ramp rate directly influences the results: a ramp rate that is too fast produces a significant error in the thermostability evaluation.
Tonset from DLS vs. SLS when comparing
Tonset from DLS and SLS reveals that the onset temperature from SLS is generally a little lower than the onset from DLS (Table 1). Figure 3 contrasts Rh and MW against temperature during 0.1 °C/minute and 2 °C/minute heating rates.
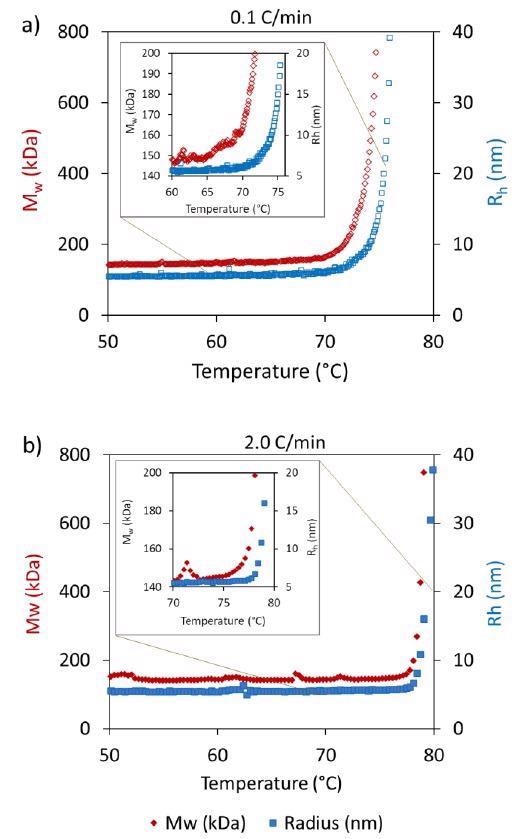
Figure 3. Differences are observed in the onsets of Mw and Rh between heating rates of a) 0.1 °C/min and b) 2.0 °C/min. Image Credit: Wyatt Technology
While the onsets at the highest ramp rate are extremely similar, there is clearly an earlier onset for Mw seen at the lowest ramp rate in contrast to Rh. SLS is generally more susceptible to aggregate formation, so even if the unfolding could not be seen using DLS, aggregation was picked up by SLS.
Additionally, it is feasible that the increase in apparent Mw without a corresponding increase in Rh is the product of transient self-association, which increases upon conformational changes that occur in the mAb that do not directly influence Rh.
Mapping ramp rate versus apparent Tonset
A possible explanation for the absence of the earlier onset at the quickest ramp rate could be that unfolding around ~71 °C results in a slower aggregation process than the one seen at what is assumed to be complete unfolding and subsequent aggregation at ~78 °C.
Therefore, the application of several heating rates between 0.1 °C/minute and 2 °C/minute was completed to establish the onsets for Rh and Mw (Table 1). Figure 4 illustrates how an apparent increase in Tonset from DLS and SLS relates to an increasing ramp rate.
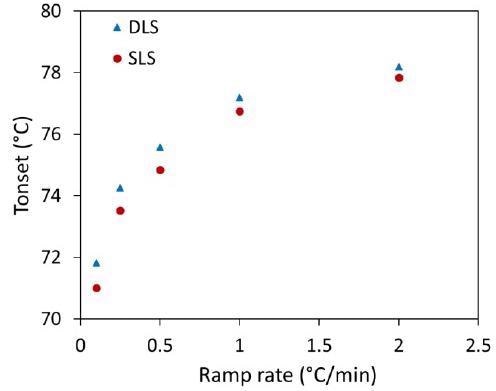
Figure 4. Influence of heating rates on Tonset of trastuzumab observed for DLS (Rh) and SLS (Mw). Image Credit: Wyatt Technology
Aggregation during a thermostability experiment takes place when unfolding uncovers hydrophobic residues generally concealed inside the protein, which (usually) leads to irreversible self-association.
The aggregation process is dependent on temperature, which indicates that as temperatures increase, the rate of aggregation increases. Based on this, the earlier onset seen during the reduced heating rates is a result of a much slower aggregation process associated with a partially unfolded protein.
When increasing the heating rate, there is a decrease in the time spent at each temperature step, mitigating the chance of aggregation occurring, while higher temperatures encourage the speed of the aggregation process.
These opposing effects result in a shift towards higher onset temperatures with increasing heating rates until the total unfolding of the mAb becomes the primary source of aggregation at the highest heating rates.
To sum up, aggregation that results from partially unfolded mAb is a decelerated process when compared to the aggregation of completely unfolded protein which explains the absence of an earlier onset at the highest ramp rates.
Conclusions
Thermostability studies are crucial for biotherapeutic characterization and the best selection of candidates and formulations. Although not typically associated with heating rate, fast ramps may conceal slow, early-onset aggregation if unfolding is a stepwise process.
Understanding how ramp rate impacts this process can be easily addressed using DLS and SLS measurements in a DynaPro NanoStar.
The NanoStar applies precise temperature control that covers an extensive temperature range from -10 °C to +120 °C yet with exceptionally low sample consumption — around just 2 µL. The instrument is the ideal instrument of choice when taking on complex and challenging thermostability studies on a limited number of samples.
For high throughput screening, the DynaPro Plate Reader can carry out thermostability testing in conventional microwell plates.
Although the DynaPro Plate Reader does not have the ability to conduct temperature ramps at the same speeds as the NanoStar, this article demonstrates that faster ramps can generate considerable miscalculations of thermal stability of mAbs and other biotherapeutics.
References
- Le Basle, Y. et al, “Physicochemical Stability of Monoclonal Antibodies: A Review”, J. Pharm. Sci. 109(10):169-190 (2020). https://doi.org/10.1016/j.xphs.2019.08.009
- AN5005: La, A. et al, “Characterization and Formulation Screening of mAb and Antibody-Drug Conjugates (ADCs) by High-throughput DLS”. https://www.wyattfiles.s3-us-west2.amazonaws.com
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.