The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which led to the COVID-19 pandemic causes mostly mild or asymptomatic disease. Still, up to 20% of infections lead to severe pneumonic illness, often complicated by multi-organ dysfunction and death. In about half of patients, gastrointestinal symptoms are present, and these patients manifest a longer disease course, with rectal swabs testing positive for viral RNA for a prolonged period after nasopharyngeal swabs became negative. Moreover, the disease is often of increased severity in this subset of patients.
It is known that the virus requires the presence of the angiotensin-converting enzyme 2 (ACE2) and Transmembrane Serine Protease 2 (TMPRSS2) on the host cell membrane in order to enter and infect the host cell successfully. However, these proteins are found in multiple organs, including the lung and the esophagus, ileum, and the colon.
Drug Testing in Human Intestinal Organoids
Human intestinal organoids (HIOs) refer to tiny masses of intestinal tissue replicating the normal function and structure of the intestine. Single-layer HIOs are derived from the human gut and have ACE2 receptors and are also susceptible to infection by SARS-CoV-2. But their architecture is simpler, and they are difficult to transplant into living organisms.
For this reason, they were replaced by HIOs derived from pluripotent stem cells, and these are now being used to test the mechanism of action and efficacy of new drugs. Remdesivir is among the very few drugs – or perhaps the only one – that is now proven to have some benefit on the pulmonary manifestations of COVID-19. Famotidine, which is a histamine-2 receptor blocker, has also been tested for its potential effect on the severity of disease, but lacks any evidence of benefit so far.
The current study uses HIOs from pluripotent stem cells to examine the ability of SARS-CoV-2 to infect specific intestinal cell types and to test the efficiency of potential drug candidates in an environment similar to the natural tissue.
Intestinal ACE1 and TMPRSS2 Expression
The results show that the human gut expresses both ACE2 and TMPRSS2, as well as the HIOs. The gut tissues with prominent ACE2 expression include the duodenal, gall bladder, and colonic epithelium, which was replicated in the HIOs. ACE2 was also found on chromogranin A (CHGA)-positive enteroendocrine and lysozyme (LYZ)-positive Paneth cells
Infected HIOs showed the presence of the viral spike protein in 10% at 24 hours, 57% at 48 hours, which indicated that the virus was both replicating and spreading to infect nearby cells. Another supporting bit of evidence came from the RT-qPCR, which showed that viral RNA was increasing in both organoids and the surrounding tissue matrix, over time.
When the tissue was stained to show the different cell types that were playing host to the virus, the researchers found that the intestinal endocrine cells which were CHGA-positive and Paneth cells that were LYZ-positive also contained viral spike protein. Goblet cells producing mucin did not stain positive for mucus but were surrounded by cells that did, which could mean that goblet cells either are not infected or abort infection.
On the other hand, the presence of cleaved caspase 3 enzyme in virally infected cells shows that these cells have entered programmed cell death, or apoptosis. As a whole, these results show that the virus is able to enter and replicate in and spread among the majority of cell types in HIOs, with the conspicuous exception of goblet cells.
Remdesivir Suppresses Viral Replication
The researchers then analyzed the effect of the presence of remdesivir and famotidine in cell cultures exposed to the virus. They found that viral replication was inhibited by 50% at a remdesivir concentration of 46 nM (IC50). At 500 nM, the drug reduced the rate of infection of the HIO by 86%, and at 5 μM, there was almost zero infection.
When famotidine was used, there was no significant effect on infection at concentrations of up to 2.5 nM in cell culture or on HIOs. The qPCR test showed these findings to be valid even with a longer duration of exposure to these drugs.
Lastly, they tested the antiviral activity of EK1, a relatively newer peptide that inhibits the membrane fusion of all coronaviruses. This molecule resulted in a reduction in the number of virally infected cells, as shown by the presence of the spike protein, by 38% at 48 hours from infection, at a concentration of 10 μM EK1.
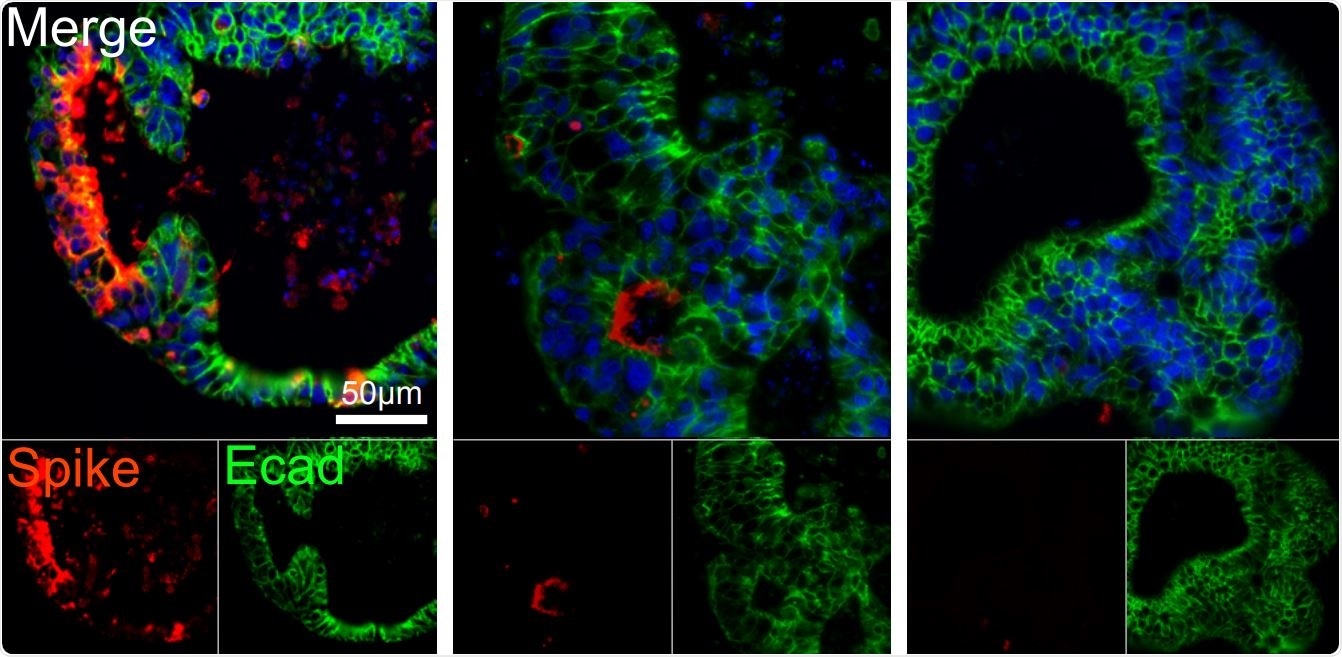
Human intestinal organoids derived from pluripotent stem cells (PSC-HIOs) express ACE2 and TMPRSS2 and support SARS-CoV-2 infection which is inhibited by remdesivir. Remdesivir treatment decreases SARS-CoV-2 infection of PSC-HIOs as shown by viral Spike protein staining 48 h post-infection. Quantification of infected, spike protein-positive cells (mean ±SEM).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Implications and Applications
These findings suggest that remdesivir could be useful to treat COVID-19 with mainly gastrointestinal manifestations. The use of HIOs that demonstrate infection of specific cells could provide a useful tool to understand how gut symptoms arise.
This study also shows for the first time that not only intestinal epithelial cells, but also intestinal endocrine cells, are susceptible to the virus. The importance of this discovery is its suggestion that the virus not only interrupts the physiological process of absorbing and transferring metabolic products from the gut but also hinders the normal endocrine regulation of the gut and its related organs.
Paneth cell infection could also worsen the results of this outcome by slowing or disrupting the secretion of antimicrobial and antiviral proteins into the gut. The long duration of disease in gastrointestinal COVID-19 and the increased severity of disease, as mentioned above, in conjunction with the known role of Paneth cells in local intestinal immunity, makes the role of drugs that are capable of inhibiting intestinal COVID-19 particularly important.
With these findings in mind, remdesivir appears to be an interesting drug to explore. However, its success is still to be established across a diverse set of patients and populations, and even across different organs of infection. The question of its efficacy has received a partial and preliminary answer as a result of the current experiment.
The researchers say, “Remdesivir inhibits SARS-CoV-2 infection of and replication in PSC-HIOs, highlighting its possible use for treatment of gastrointestinal infection occurring simultaneously with or independently of respiratory manifestation of COVID-19.”
On the other hand, famotidine doesn’t affect the rate of intestinal infection with SARS-CoV-2. Future research must focus on establishing the results of long-term treatment and use a prospective model of testing.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources