After collection from an individual, how are COVID-19 sample swabs transported to the laboratory?
Once a sample swab has been collected from the individual, it is placed into a transport tube containing a form of transport media; alternatively, swabs can be transported dry.
The method of transportation chosen will have a significant impact on sample RNA integrity and stability. An individual’s sample is then transported to a test laboratory via the postal system or through a designated courier. Therefore, it is critical to consider the safety of all individuals that come into contact with the hazardous sample.
Molecular transport media (MTM) offers the safest option for transportation as it deactivates the virus, whereas samples stored in generic transport medias will remain infectious and continue to pose inherent safety risks.
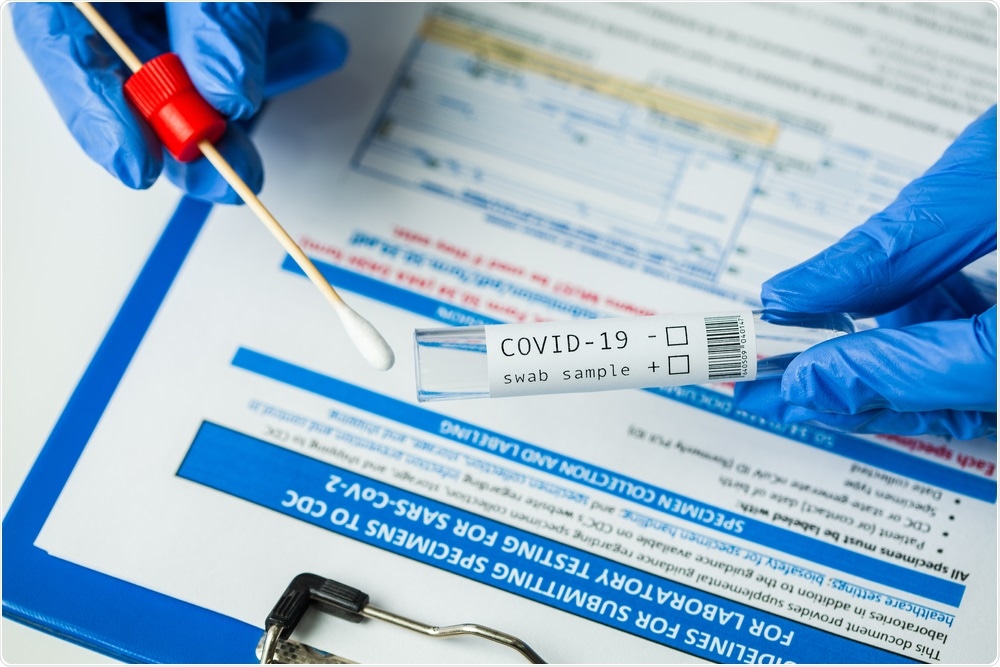
Image Credit: Cryptographer/Shutterstock.com
What is PrimeStore Molecular Transport Media, and how does it differ from other transportation medias?
PrimeStore® MTM (Molecular Transport Medium) is a Class 2 medical device that was designed and optimized for molecular testing. The media inactivates microbes whilst preserving and stabilizing the released DNA and RNA for downstream molecular applications, including next-generation sequencing and qPCR.
In comparison, generic universal transport medias (UTMs) were designed for transporting intact viruses for culture work. PrimeStore MTM ensures sample stability for up to seven days at ambient temperatures meaning cold chain transportation and storage are not required.
Unlike most transport medias, PrimeStore MTM inactivates the virus within 60 minutes of collection, eliminating the risks to safety during the transportation and sample analysis process.
Why is it important to remove the need for the cold chain when transporting samples?
Cold chain adds significant costs and difficulties to the sample collection process and is not always a viable option. For example, as home testing becomes more readily available, increasing numbers of samples are being transported through the postal system, where cold chain is not an option.
The UK Government’s recently stated target of completing 500,000 tests for COVID-19 per week highlights the importance of the ability to transport samples quickly and safely.
Using PrimeStore MTM for viral transport, can the sample be used for multiple tests? Or do multiple swabs need to be taken?
Multiple tests can be processed from one sample swab. This makes PrimeStore MTM ideal for the flu season as samples stored in PrimeStore MTM can be tested for both COVID-19 and influenza from a single sample swab.
PrimeStore MTM has been designed for short term and long-term sample storage meaning it is possible to bio-bank samples in the collection tube for future analysis.
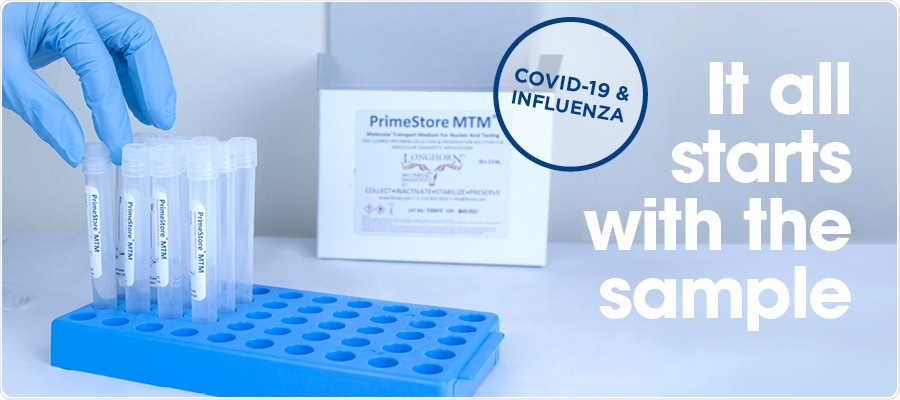
Image Credit: https://www.ekfdiagnostics.com/PrimeStore-MTM.html
What is the future for the PrimeStore MTM and the viral transport media from EKF Diagnostics?
We think that, in time, people will look back and wonder why health systems ever used viral transport methods that required samples containing a live virus to be sent around countries and sent to labs – especially when methods that can deactivate the virus and preserve the RNA and DNA were openly available and molecular testing is predominantly used.
In the meantime, we have launched PrimeStore MTM tubes as part of a bulk sample collection kit, which is suitable for mass on-site sample collection. The kit pairs the PrimeStore MTM sample inactivation media with a nasopharyngeal flocked swab in a CE marked kit.
What does EKF Diagnostics offer as an alternative to general viral transport media brands? What are the key features that differentiate this viral transport media from others?
PrimeStore MTM is a patented, FDA cleared and CE-IVD marked molecular transport media that has been designed and optimized to suit the evolving requirements of sample collection, transportation, and analysis. PrimeStore MTM inactivates the sample within 60 minutes of collection allowing samples to be safely transported and analyzed outside of category III containment.
Additionally, samples stored in PrimeStore MTM are stable for seven days at ambient temperatures (2-15°C) or 30 days at 2-8°C or can be bio-banked for long term storage. Each batch of PrimeStore MTM is formulated with an internal positive control (IPC) nucleic acid comprising a specific sequence that can be used to monitor sample integrity and preservation from the collection, extraction through detection.
There are many other brands of VTM and UTM available on the market. None, however, offer the same features with the back up of FDA Class 2 clearance and CE IVD mark.
Are the ingredients of the PrimeStore MTM detectable in subsequent molecular testing? If so, can this inhibit molecular testing?
Unlike generic viral transport media, PrimeStore MTM has been developed and optimized for molecular testing and therefore does not contain any components that may inhibit PCR testing.

Image Credit: Cryptographer/Shutterstock.com
What does FDA Class 2 cleared mean for your PrimeStore MTM?
PrimeStore MTM has been FDA cleared which means it has undergone a 510K submission that the FDA has reviewed, allowing the device to be marketed and sold within the US. FDA Class 2 clearance requires a greater level of testing and scrutiny to be applied before it is granted. PrimeStore MTM is the only viral transport media to be able to make this claim.
Where can readers find more information?
Take a look at the EKF Diagnostics website (www.ekfdiagnostics.com) for more information about PrimeStore MTM. We have collated information that answers most people’s questions as well as MSDS and a list of studies where PrimeStore MTM has been used including a publication from Public Health England.
About Rebekah Stibbs
Rebekah Stibbs has worked within the Life Science industry for the past seven years. Rebekah began her career as a laboratory apprentice and progressed to obtain roles within quality control and Research and Development labs whilst studying with the Open University.
More recently she has left the laboratory environment to progress to become a Product Manager at EKF Diagnostics.