With increasing numbers of people suffering from coronavirus disease 2019 (COVID-19), and most recovering, the levels of protective immunity need to be measured over time to help measure the duration of protective immunity.
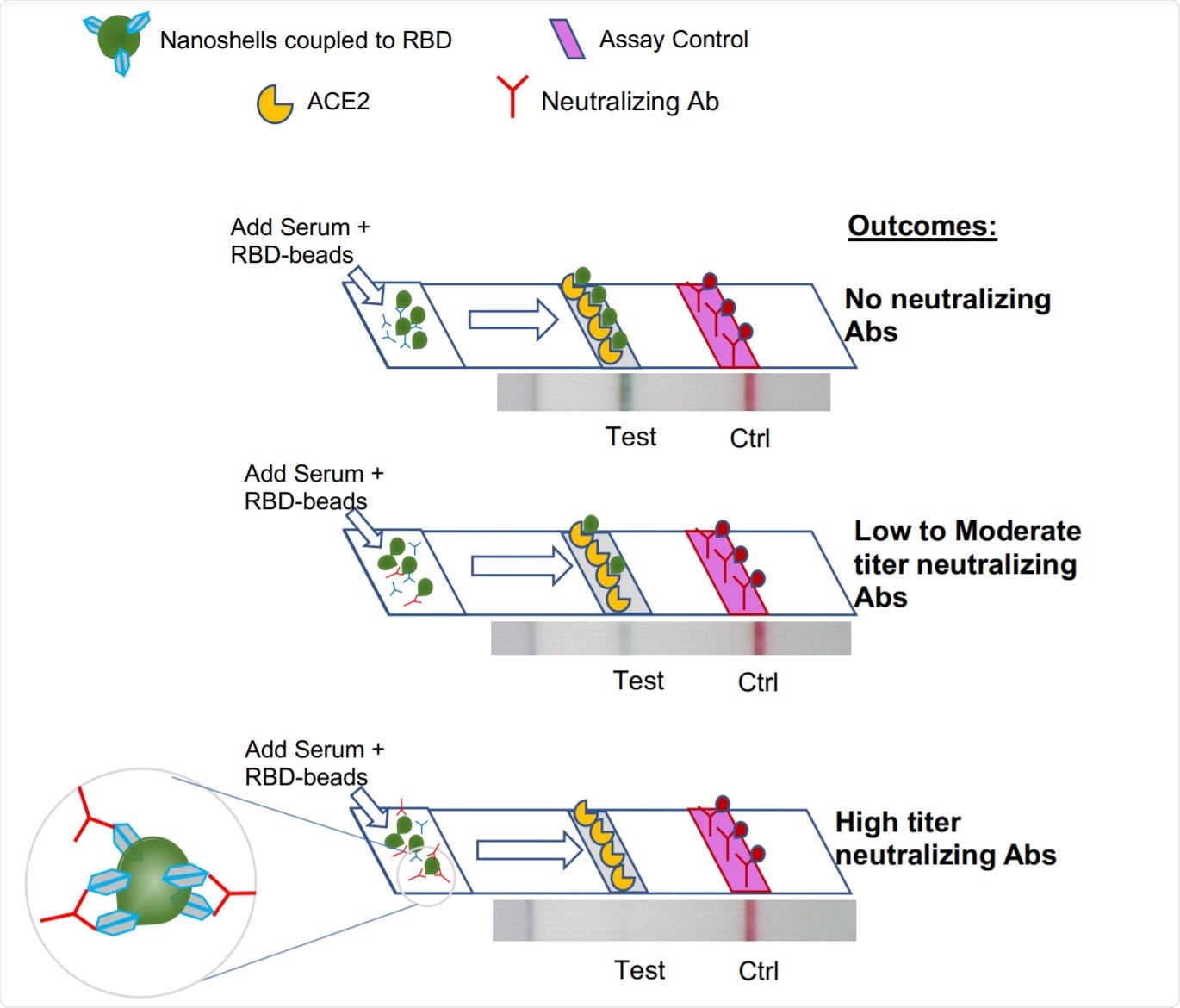
Schematic of Neutralization LFA. Image Credit: https://www.medrxiv.org/content/10.1101/2020.12.15.20248264v1.full.pdf

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The need for neutralization assays
Many molecular tests for viral nucleic acid, such as polymerase chain reaction (PCR), are meant to diagnose the presence of the virus, and thus, of infection or re-infection. They also indicate virus clearance in recovering patients.
All patients with a healthy immune system respond to the causative severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by producing antibodies. Early in the recovery phase, therefore, patients can have both a positive PCR and a positive antibody test, which shows that the presence of antibodies is not always a pointer to recovery in the case of SARS-CoV-2 infection.
A wide range of antibodies is generated against the virus, but all are not neutralizing. Thus, individuals who have neutralizing antibodies (NAbs) need to be identified to return to work, for instance, with little risk of re-infection or of transmitting the virus to others.
The same applies to the probable increase in vaccine coverage. And thirdly, these individuals are probably the best donors of convalescent plasma (CP) for the treatment of patients with severe COVID-19. This is highlighted by the fact that in a third of CP units from COVID-19 convalescents, NAbs are absent.
Current neutralizing assays
The neutralizing activity of serum is measured by viral neutralization assays, of which there are two types. The first assesses the extent to which viral plaques or foci of infection in host cells are reduced, in microneutralization assays, using wildtype SARS-CoV-2. This type of study can be carried out only in biosafety level 3 (BSL3) laboratories. Moreover, this is a slow and cumbersome procedure, requiring much skill.
The other consists of pseudovirus neutralization assays. A pseudovirus is a virus such as lentivirus in which SARS-CoV-2 spike protein is expressed. Since they do not use the wild-type virus, they can be carried out under BSL2 conditions. Still, the results can take up to 24-48 hours. With both these assays, the type of host cell impacts the level of infection, making the results more variable.
Lateral flow assay – rapid and accurate
The current paper describes a lateral flow assay (LFA) that evaluates the levels of NAbs preventing the attachment of the viral receptor-binding domain (RBD) on the spike glycoprotein to the host cell receptor, the angiotensin-converting enzyme 2 (ACE2). This test is a rapid assay, with the results being available in 10 minutes. It can use serum, plasma, or whole blood.
The researchers tested serum known to have NAbs as well as clinical serum samples. They found that the LFA correlated correctly with serum titers, and was most accurate at higher titers. It accurately identified serum with non-neutralizing activity.
They then tested another 38-sample set that had known half-maximal inhibition values (IC50), as measured by a wildtype virus neutralization assay. This blinded assay also showed that strong neutralization activity in the assay was correlated with low RBD-ACE2 binding in the LFA.
Thirdly, the researchers explored the presence of cross-neutralization activity in serum from patients with other respiratory viruses. Even though the other pathogens included the seasonal coronaviruses, none of the samples had neutralizing activity against SARS-CoV-2.
Comparable with FDA standard
They also compared the results of the Ortho assay and their LFA in measuring neutralizing activity. According to the Food and Drug Administration (FDA), an Ortho VITROS SARS-CoV-2 IgG assay of 12 or above is required for the use of plasma as CP in COVID-19 patients. This comparison showed that about half and a little above a quarter of observed IC50 variance was contributed by LFA and Ortho VITROS. LFA values showed a negative correlation with the IC50 values, and the latter showed a positive correlation, both being positive.
Both assays agreed well with the titer, but the LFA classified neutralizing samples more accurately, at a titer of 1:320 or above, with no tendency to over-or under-estimate IC50 values.
What are the implications?
The test is rapid, reliable, cheaper than comparable tests, highly portable, and semi-quantitative, and can be used as a point-of-care (POC) diagnostic test. It can be used to assess the duration of protection of NAbs following natural infection or vaccination since only a fingerstick is needed rather than a blood draw. It could also be useful to measure the level at which NAbs prevent re-infection and viral transmission.
Additionally, it could be used to achieve a rapid and useful classification of CP by neutralizing activity, so that those with the most severe disease could receive CP with the highest neutralization activity.
The researchers demonstrated this by measuring the conversion of ordinary non-immune plasma into plasma with strong neutralizing activity by the addition of small amounts of highly neutralizing plasma.
This test could also be used to monitor the appearance of NAbs in patients who have received CP with high neutralizing activity, helping to decide whether another unit with similar activity, or hyperimmune IgG, or neutralizing monoclonal antibodies, are required. This could also be of use in clinical trials employing these treatment modalities.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources