Vaccination has been deemed the only effective way to contain the ongoing coronavirus disease 2019 (COVID-19) pandemic, which is caused by the rapid outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Several vaccines have received approval from various regulatory bodies across the world, which has led to widespread vaccination campaigns in these nations.
As vaccines become more readily available to the global population and life begins to return to normal, it is crucial to understand the strength of the protective humoral immunity induced by both natural SARS-CoV-2 infection and COVID-19 vaccines.
 Study: Homologous and Variant-Specific Memory B-Cell and Antibody Responses after SARS-CoV-2 mRNA Vaccination. Image Credit: insta_photos / Shutterstock.com
Study: Homologous and Variant-Specific Memory B-Cell and Antibody Responses after SARS-CoV-2 mRNA Vaccination. Image Credit: insta_photos / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Memory B cells and SARS-CoV-2
The longevity of the immune response to SARS-CoV-2 infection is largely dependent on the synthesis and persistence of antigen-specific isotype-switched memory B cells (MBCs) and long-lived plasma cells present in the bone marrow.
The most dominant immune isotype of MBCs after COVID-19 infection is immunoglobulin G (IgG). The presence of receptor-binding domain (RBD)-specific to IgG serves as a marker of immune memory cells induced post-SARS-CoV-2 infection or vaccination.
To date, the effectiveness of messenger ribonucleic acid (mRNA) vaccine-induced MBCs against the SARS-CoV-2 variants of concern (VoCs) has not been determined. As a result, scientists around the world are looking to better understand the efficacy of vaccine-induced immune MBCs against SARS-CoV-2 VoCs and their impact on the longevity of heterotypic protection.
About the study
A new study published on the preprint server medRxiv* characterizes the functional humoral immunity post-COVID-19 mRNA-based vaccination, as well as the reactivity of COVID-19 vaccine-induced MBCs against the SARS-CoV-2 VoCs.
To do this, the blood samples of 17 healthy subjects, among which 15 were naïve and 2 had previously recovered from COVID-19 infection, were analyzed prior to their vaccination using the BNT162b2 (Pfizer/BioNtech) or mRNA-1273 (Moderna) vaccine.
Blood samples were again collected from the same subjects twice post-vaccination. The two subsequent time points were three weeks after receiving the first dose and two weeks after receiving the second dose.
Study findings
Most of the subjects who participated in this study received the Pfizer-BioNTech vaccine (BNT162b2), while only three participants received the Moderna mRNA-1273 vaccine.
To study the longevity of the effectiveness of the vaccines, the authors developed a profile based on the frequencies and specificities of isotype-switched IgG+ MBCs after SARS-CoV-2 mRNA vaccination. This profile would indicate the presence of mature, specific, and functional immune memory cells.
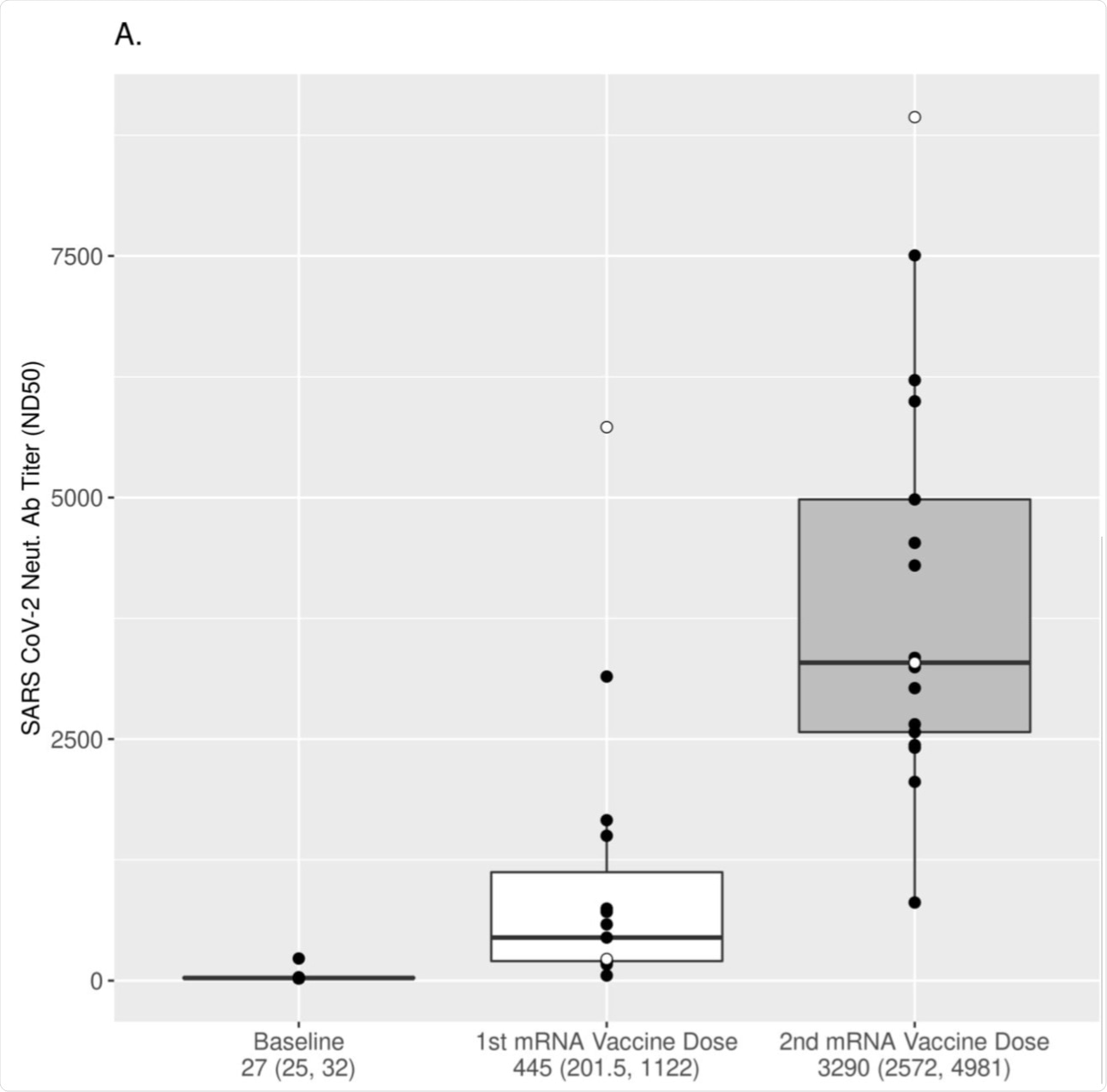 (A) The dynamics of neutralizing antibody response after vaccination was assessed using a pseudovirus microneutralization assay (see Methods). ND50 values over three time points relative to vaccination are displayed using boxplots for all subjects. The values are plotted in log2 scale, but the scales of the axis reflect the untransformed values for easier interpretation. Each box was plotted using the 25% to 75% interquartile range and the median is represented by the bold line in the box. The “ whiskers” extend up to 1.5 times the interquartile range above or below the 75th or 25th percentiles, respectively. Black dots represent naïve (at baseline) subjects, while white dots represent COVID-19-recovered (at baseline) subjects. The numbers below the x-axis represent the median response for all subjects (25% and 75% interquartile range). Titers increased significantly after each vaccine dose (p-value<0.001 for all comparisons, Wilcoxon signed-rank test).
(A) The dynamics of neutralizing antibody response after vaccination was assessed using a pseudovirus microneutralization assay (see Methods). ND50 values over three time points relative to vaccination are displayed using boxplots for all subjects. The values are plotted in log2 scale, but the scales of the axis reflect the untransformed values for easier interpretation. Each box was plotted using the 25% to 75% interquartile range and the median is represented by the bold line in the box. The “ whiskers” extend up to 1.5 times the interquartile range above or below the 75th or 25th percentiles, respectively. Black dots represent naïve (at baseline) subjects, while white dots represent COVID-19-recovered (at baseline) subjects. The numbers below the x-axis represent the median response for all subjects (25% and 75% interquartile range). Titers increased significantly after each vaccine dose (p-value<0.001 for all comparisons, Wilcoxon signed-rank test).
To this end, a significant change in the antigen-specific MBCs was observed over time post-vaccination. More specifically, significant SARS-CoV-2-specific MBCs were identified in response to the S1 portion of the SARs-CoV-2 spike (S) protein, particularly to its RBD, of the original SARS-CoV-2 strain. This same immune response was further boosted after the participants received the second booster dose.
When evaluated against the RBDs of clinically significant VoCs, the researchers found that RBD-specific MBCs were readily detectable against all variant RBDs following mRNA vaccination. However, following the second vaccine dose, the researchers found a significant decrease in the number of reactive MBCs capable of recognizing the Beta, Gamma, and Delta RBDs. Moreover, the difference in the MBC response to the Alpha variant was not found to be significant.
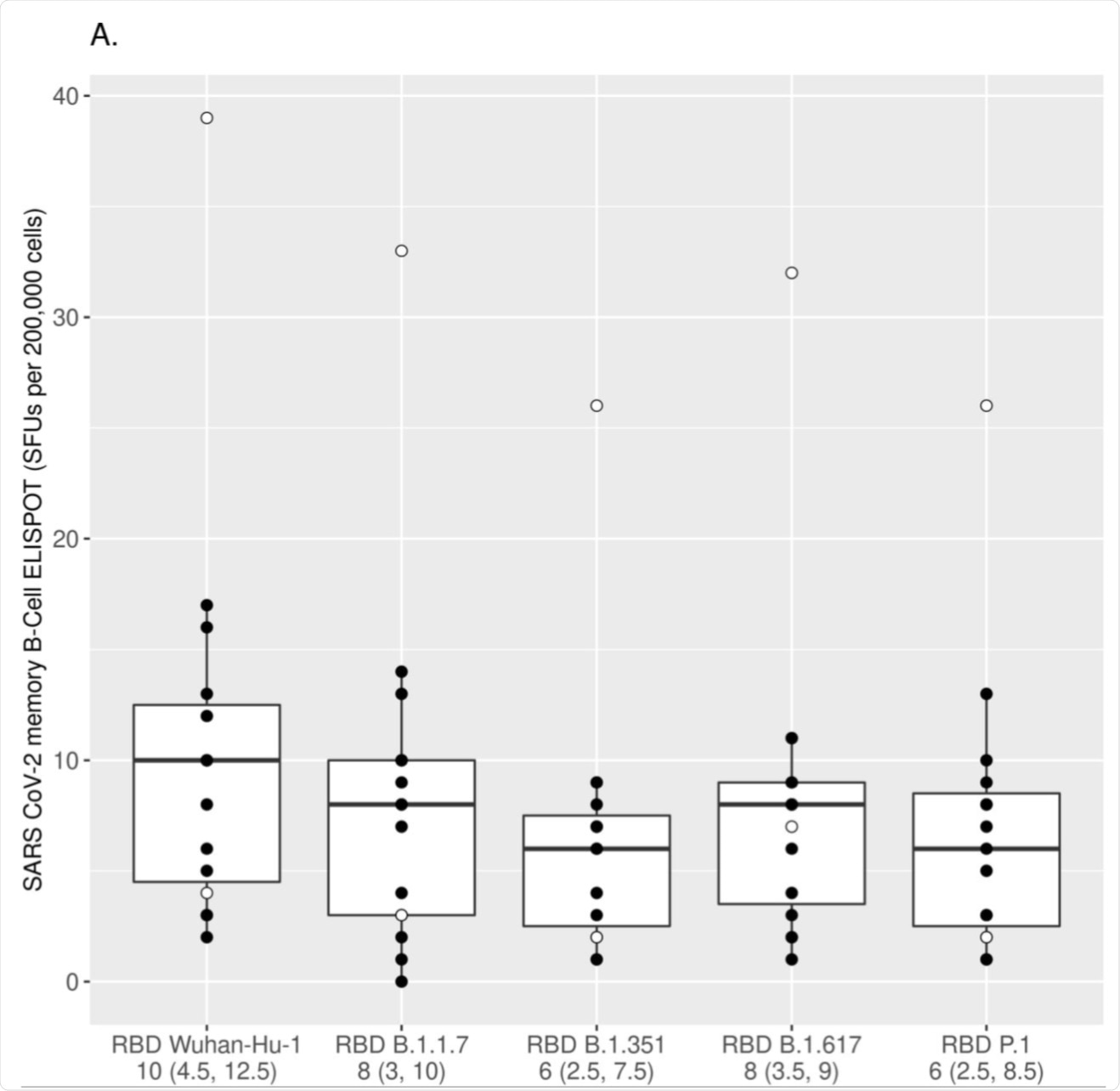
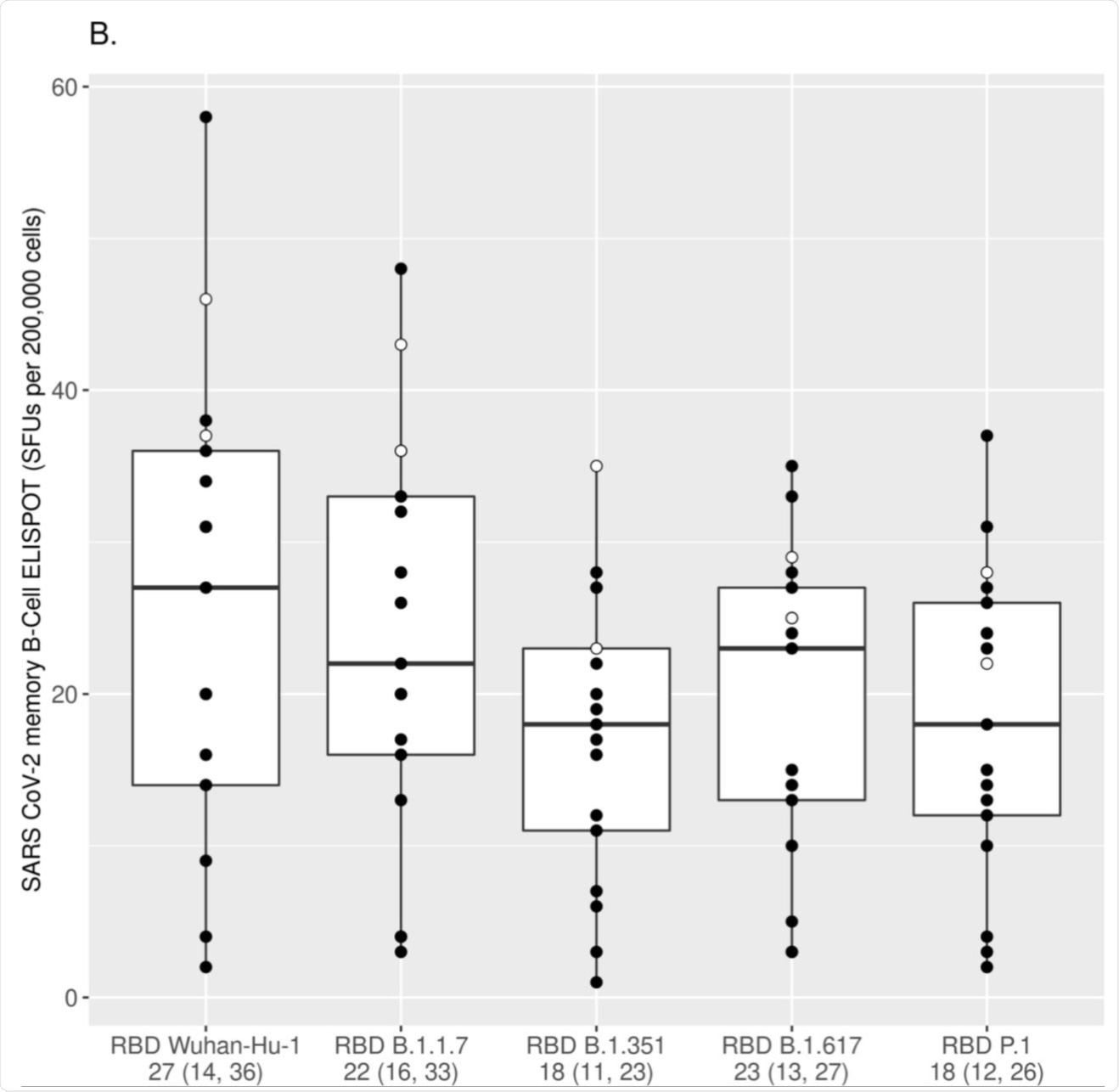 The numbers of IgG+ MBCs reactive to the Wuhan-Hu-1 RBD and the RBDs of different VoC strains (Alpha/RBD B.1.1.7, Beta/RBD B.1.351, Gamma/RBD P.1 and Delta/RBD B.1.617) were measured using ELISPOT assay and assessed after the first mRNA vaccine dose (A) and after the second mRNA vaccine dose (B). Each box was plotted using the 25% to 75% interquartile range and the median was represented by the bold line in the box. The “ whiskers” extend up to 1.5 times the interquartile range above or below the 75th or 25th percentiles respectively. Black circles represent naïve (at baseline) subjects, while white circles represent COVID-19-recovered (at baseline) subjects. The numbers below the x-axis represent the median response for all subjects to the specified RBD antigen (25% and 75% interquartile range/IQR). The MBC response to each variant RBD was compared to the response directed to the Wuhan-Hu-1 RBD using the Wilcoxon signed-rank test [p<0.025 for all comparisons after the first dose (A); p<0.001 for all comparisons after the second dose (B) with the exception of Alpha/RBD B.1.1.7, which was non-significant after the second dose].
The numbers of IgG+ MBCs reactive to the Wuhan-Hu-1 RBD and the RBDs of different VoC strains (Alpha/RBD B.1.1.7, Beta/RBD B.1.351, Gamma/RBD P.1 and Delta/RBD B.1.617) were measured using ELISPOT assay and assessed after the first mRNA vaccine dose (A) and after the second mRNA vaccine dose (B). Each box was plotted using the 25% to 75% interquartile range and the median was represented by the bold line in the box. The “ whiskers” extend up to 1.5 times the interquartile range above or below the 75th or 25th percentiles respectively. Black circles represent naïve (at baseline) subjects, while white circles represent COVID-19-recovered (at baseline) subjects. The numbers below the x-axis represent the median response for all subjects to the specified RBD antigen (25% and 75% interquartile range/IQR). The MBC response to each variant RBD was compared to the response directed to the Wuhan-Hu-1 RBD using the Wilcoxon signed-rank test [p<0.025 for all comparisons after the first dose (A); p<0.001 for all comparisons after the second dose (B) with the exception of Alpha/RBD B.1.1.7, which was non-significant after the second dose].
Conclusion
The current exploratory study reported the effectiveness of mRNA vaccines against the three VoCs; namely, the Alpha, Beta, and Gamma SARS-CoV-2 variants. Post mRNA vaccination, a reduced functional antibody response to the VoCs, particularly for the Beta and Gamma variants, was found.
In line with previous studies, this research has also revealed a strong S1/RBD-dominated MBC response post-vaccination, which was further enhanced after the second dose of the vaccine. To the authors’ knowledge, differential specificity of mRNA vaccine-induced MBC response to multiple emerging VoC RBDs was reported here for the first time.
As the results presented here were acquired from only 17 individuals, the authors emphasized the need to further evaluate the effectiveness of all the available COVID-19 vaccines against the newly emerging SARS-CoV-2 VoCs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Haralambieva, H. I., Monroe, J. M., Ovsyannikova, I. G., et al. (2021). Homologous and Variant-Specific Memory B-Cell and Antibody Responses after SARS-CoV-2 mRNA Vaccination. medRxiv. doi:10.1101/2021.07.12.21260386. https://www.medrxiv.org/content/10.1101/2021.07.12.21260386v1
- Peer reviewed and published scientific report.
Haralambieva, Iana H, Jonathon M Monroe, Inna G Ovsyannikova, Diane E Grill, Gregory A Poland, and Richard B Kennedy. 2022. “Distinct Homologous and Variant-Specific Memory B-Cell and Antibody Response over Time after Severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA Vaccination.” The Journal of Infectious Diseases 226 (1): 23–31. https://doi.org/10.1093/infdis/jiac042. https://academic.oup.com/jid/article/226/1/23/6524526.