CAR-T therapies are a new successful strategy for treating hematological malignancies. However, recent studies have demonstrated the limitations of such therapies, such as the occurrence of neurotoxicity, graft-versus-host disease (GVHD), and cytokine release syndromes (CRS).
This has motivated researchers to seek alternative, safer cells, such as natural killer (NK) cells.1 NK cells are a type of lymphocyte that destroys tumor cells and virus-infected cells non-specifically, without prior sensitization.
CAR-NK therapy entails NK cells being modified with mature chimeric antigen receptor (CAR) technology to take advantage of their broad tumor-killing ability, as well as their unique target cell recognition mechanism. The CAR-NK therapeutic strategy is presented in Figure 1.1
CAR-NK therapy offers many benefits that CAR-T therapy does not, including significantly reduced CRS toxicity and immune effector cell-associated neurotoxicity syndrome toxicity, as well as lower GVHD, which improves the overall safety of the treatment.2, 3
Additionally, NK cells have multiple tumor recognition sites, which potentially reduces the occurrence of antigenic escape failure4.
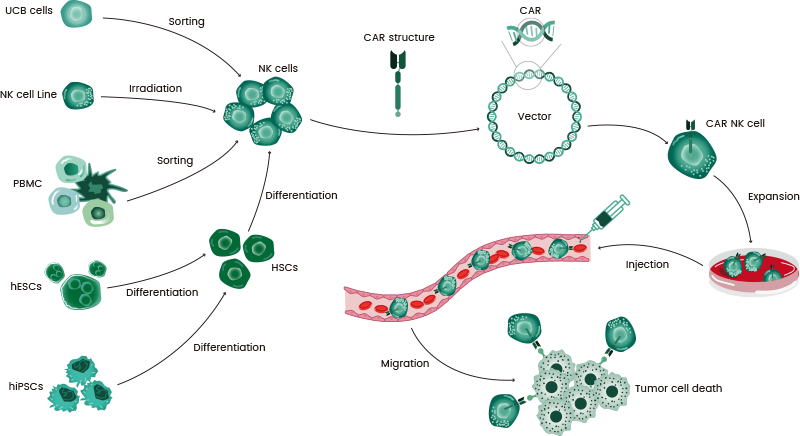
Figure 1. The flow chart of CAR-NK therapy. Image Credit: Sino Biological Inc.
CAR molecule design
CAR has the capacity for the specific recognition of target cells and the activation of NK cells to destroy tumor cells.
As a result, the CAR structure’s design and engineering are critical for CAR-NK therapeutic success. Like CAR-T, CAR is comprised of a hinge domain, an extracellular antigen binding domain, an intracellular domain, and a transmembrane (TM) domain.2
The extracellular domain has a single-chain variable fragment (scFv) that is derived from monoclonal antibodies and enables the ability to specifically recognize and bind to tumor antigens. The hinge domain connects the extracellular domain to the TM domain.5
The intracellular signaling domain defines the activation signal’s strength and directly influences the tumor-killing effect.6 A summary of the molecules of CAR structure in CAR-NK is presented in Table 1.2
Table 1. Molecules of the CAR structure in CAR-NK. Source: Sino Biological Inc.
| Domains |
Molecules |
Extracellular
domain |
VH-VL, VL-VH, VH-only |
Hinge
domain |
CD8a, CD28, DAP12, IgG4, IgG2 CH2-CH3, IgG1 CH2-CH3, IgG4 CH2-CH3 |
TM
domain |
CD3ζ, CD28, CD8a, 4-1BB, DAP12, TCR ab, CD28-CD3ζ, FceRIγ, murine CD3ζ |
Intracellular
domain |
CD3ζ, CD28, 4-1BB |
There are currently four generations of CARs, with various structures and compositions, as displayed in Figure 2. First-generation CARs contain only DAP12 or CD3ζ as signaling structural domains.
A second signaling structural domain is expressed by second-generation CARs, including 4-1BB or CD28 associated with CD3ζ, while third-generation CARs involve CD3ζ and two co-stimulatory signaling domains (4-1BB and CD28).
Lastly, fourth-generation CARs involving NK cells can release cytokines, which allow the regulation of NK cell proliferation. These cytokines include IL-2 and IL-15. This effectively improves the anti-tumor capability of NK cells against lymphoma xenografts.1,7
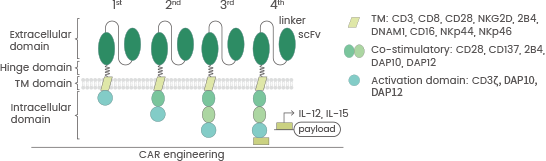
Figure 2. The four generations of CAR structure. doi:10.1186/s40364-022-00364-6. Image Credit: Sino Biological Inc.
Source of NK cells
NK-92 cell lines are widely employed as a source of CAR-NK cells because of their significant proliferation ability in vitro and decreased sensitivity to repeated freeze/thaw cycles.
However, NK92 cells are malignant cells from an NK cell lymphoma, and they present some limitations, such as the potential risk of tumorigenicity, the absence of NKp44 and CD16 expression, and the requirement for lethal irradiation before infusion, which hinders the potential of in vivo expansion.
An alternative source of NK cells is umbilical cord blood (UCB), which comes with various benefits, including low T cell contamination risk, off-the-shelf availability, and great promise for proliferation and persistence in vivo.
To address limitations concerning volume, UCBs are expanded to large numbers via a range of techniques, including cytokine mixture and artificial antigen-presenting cells.
Additionally, NK cells may be obtained from other sources (as displayed in Figure 1), such as cord blood (CB), peripheral blood (PB), peripheral blood mononuclear cells (PBMCs), and stem cells, including induced pluripotent stem cells (iPSCs).8, 9
Research is ongoing regarding the utilization of NK cells, with scientists seeking the best source for NK-cell immunotherapy for their research.
Preparation and expansion of CAR-NK cells
The advancements in genetic engineering technology have introduced several techniques for the generation of CAR-NK cells. CAR-NK transduction systems that are commonly used utilize both viral and non-viral vectors.
Viral vectors include lentiviruses, retroviruses, and adeno-associated viruses (AAV),6 with retroviral transduction being the most widely utilized approach for delivering CAR to NK cells.
Non-viral vectors include the PiggyBac and sleeping beauty transposons. Additionally, mRNA electroporation and the CRISPR/Cas9 method may also be employed.
Multiple methods may be employed for the expansion of CAR-NK cells, with the two most common approaches involving feeder cells and cytokine mixtures. Cytokines are vital for the in vivo development and survival of NK cells.
IL-21 promotes the proliferation of NK cells and improves their cytotoxic function, while IL-15 initiates the expansion and cytotoxic activity of NK cells. The latter is also utilized together with IL-2 to expand NK cells in vitro.10, 11
Sino Biological produces GMP-grade cytokines, such as IL-2, IL-12, IL-15, and IL-21, and offers comprehensive solutions for CAR-NK therapy to support cellular immunotherapy.
Clinical trials of CAR-NK
Multiple clinical trials of treating hematological and solid tumors with CAR-NK therapies have been carried out globally as of August 2023. There are currently no FDA-approved drugs for CAR-NK, with most presently in preclinical phases and several having entered clinical phase I/II (as shown in Table 2).
A CAR-NK therapy was recently established for targeting CD19 to treat B-cell lymphoblastic leukemia/lymphoma in a phase I/II study (NCT05654038).
This trial is a single-center, open-labeled, single-arm, non-randomized investigator-initiated trial to assess the safety and effectiveness of anti-CD19 universal CAR-NK (UCAR-NK) cells therapy in conjunction with HSCT for treating B-cell hematologic malignancies.
Additionally, the goal of another clinical trial (NCT05528341) is the evaluation of the effects and safety of NKG2D-CAR-NK92 infusion for treating relapsed/refractory solid tumors.
The clinical trials that involve CAR-NK therapy have largely focused on hematologic malignancies, such as acute myeloid leukemia (AML), B-cell lymphoma, B-cell non-Hodgkin lymphoma (B-cell NHL), acute lymphoblastic leukemia, B-cell hematologic malignancies, and multiple myeloma.12-14
However, CAR-NK therapy is also being tested for the treatment of solid tumors, including breast cancer, glioblastoma, colorectal cancer, small cell lung cancer, ovarian cancer, and prostate cancer.11, 15, 16
These clinical trials have demonstrated that CAR-NK therapies target BCMA, CD7, CD19, CD22, CD33, CD70, HER2, NKG2D, ROBO1, DLL3, mesothelin, Claudin 6, or PSMA to achieve therapeutic efficacy13, 16.
Table 2. Clinical Trials involving CAR-NK cell therapy. Source: Sino Biological Inc.
| Clinical trial |
Target |
Disease |
Cell source |
Status |
Phase |
| NCT05182073 |
BCMA |
Multiple Myeloma
Myeloma |
iPSCs |
Recruiting |
Phase 1 |
| NCT05842707 |
CD19
CD70 |
Refractory or Relapsed B-cell
Non-Hodgkin Lymphoma |
CB |
Recruiting |
Phase 1
Phase 2 |
| NCT02944162 |
CD33 |
Acute Myelogenous Leukemia
Acute Myeloid Leukemia
Acute Myeloid Leukemia With Maturation |
NK-92 cell line |
Unknown |
Phase 1
Phase 2 |
| NCT03940833 |
BCMA |
Multiple Myeloma |
NK-92 cell line |
Unknown |
Phase 1
Phase 2 |
| NCT05654038 |
CD19 |
B-Cell Lymphoblastic
Leukemia /Lymphoma |
N/A |
Recruiting |
Phase 1
Phase 2 |
| NCT04324996 |
NKG2D
ACE2 |
COVID-19 |
CB |
Unknown |
Phase 1
Phase 2 |
| NCT05645601 |
CD19 |
Adult Relapsed/Refractory B-cell
Hematologic Malignancies |
N/A |
Recruiting |
Phase 1 |
| NCT05472558 |
CD19 |
B-cell Non Hodgkin Lymphoma |
CB |
Recruiting |
Phase 1 |
| NCT05667155 |
CD19, CD70 |
B-cell Non Hodgkin Lymphoma |
CB |
Recruiting |
Phase 1 |
| NCT05008536 |
BCMA |
Multiple Myeloma, Refractory |
CB |
Recruiting |
Early Phase 1 |
| NCT03692637 |
Mesothelin |
Epithelial Ovarian Cancer |
PBMCs |
Unknown |
Early Phase 1 |
| NCT05507593 |
DLL3 |
SCLC, Extensive Stage |
N/A |
Recruiting |
Phase 1 |
| NCT03415100 |
NKG2D |
Solid Tumor |
N/A |
Unknown |
Phase 1 |
| NCT04847466 |
PD-L1 |
Gastroesophageal Junction (GEJ) Cancers
Advanced HNSCC |
N/A |
Recruiting |
Phase 2 |
| NCT05528341 |
NKG2D |
Relapsed/Refractory Solid Tumors |
NK-92 cell line |
Recruiting |
Phase 1 |
| NCT03940820 |
ROBO1 |
Solid Tumor |
Primary NK cells |
Unknown |
Phase 1
Phase 2 |
| NCT05410717 |
Claudin6 |
Stage IV ovarian cancer
Testis cancer
Refractory endometrial cancer |
PB |
Recruiting |
Phase 1
Phase 2 |
Conclusion
CAR-NK cells possess unique advantages over CAR-T cells, including more cell sources, more precise killing of tumor cells, and higher efficacy against solid tumors. However, several challenges persist, such as low transfection efficiency, cytotoxicity, and storage issues.
As the development and refining of CAR-NK therapies continue, preclinical studies and preliminary clinical data suggest that CAR-NK therapy has enhanced safety.
CAR-NK therapy lacks human leukocyte antigen matching limitations and possesses lower neurotoxicity, CRS, and GVHD, as well as the potential of using allogeneic NK cells as a CAR platform for “off-the-shelf” therapy.
As a result, CAR-NK therapy has great potential as a novel cancer treatment strategy with fewer side effects.
References
- Marofi F, Saleh MM, Rahman HS, et al. CAR-engineered NK cells; a promising therapeutic option for treatment of hematological malignancies. Stem Cell Res Ther. 2021;12(1):374. Published 2021 Jul 2. doi:10.1186/s13287-021-02462-y
- Pang Z, Wang Z, Li F, Feng C, Mu X. Current Progress of CAR-NK Therapy in Cancer Treatment. Cancers (Basel). 2022; 14(17):4318. Published 2022 Sep 2. doi:10.3390/cancers14174318
- Valeri A, García-Ortiz A, Castellano E, et al. Overcoming tumor resistance mechanisms in CAR-NK cell therapy. Front Immunol. 2022;13:953849. Published 2022 Aug 3. doi:10.3389/fimmu.2022.953849
- Liu E, Marin D, Banerjee P, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6):545-553. doi:10.1056/NEJMoa1910607
- Fujiwara K, Tsunei A, Kusabuka H, Ogaki E, Tachibana M, Okada N. Hinge and Transmembrane Domains of Chimeric Antigen Receptor Regulate Receptor Expression and Signaling Threshold. Cells. 2020;9(5):1182. Published 2020 May 9. doi:10.3390/cells9051182)
- Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14(1):73. Published 2021 May 1. doi:10.1186/s13045-021-01083-5
- Zhang L, Meng Y, Feng X, Han Z. CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomark Res. 2022;10(1):12. Published 2022 Mar 18. doi:10.1186/s40364-022-00364-6
- Zhao X, Cai L, Hu Y, Wang H. Cord-Blood Natural Killer Cell-Based Immunotherapy for Cancer. Front Immunol. 2020;11:584099. Published 2020 Oct 22. doi:10.3389/fimmu.2020.584099
- Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975. doi:10.1016/j.ebiom.2020.102975
- Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front Immunol. 2019;10:1205. Published 2019 May 31. doi:10.3389/fimmu.2019.01205
- Kilgour MK, Bastin DJ, Lee SH, Ardolino M, McComb S, Visram A. Advancements in CAR-NK therapy: lessons to be learned from CAR-T therapy. Front Immunol. 2023;14:1166038. Published 2023 May 2. doi:10.3389/fimmu.2023.1166038
- Huang R, Wen Q, Zhang X. CAR-NK cell therapy for hematological malignancies: recent updates from ASH 2022. J Hematol Oncol. 2023;16(1):35. Published 2023 Apr 7. doi:10.1186/s13045-023-01435-3
- Shao R, Li Z, Xin H, et al. Biomarkers as targets for CAR-T/NK cell therapy in AML. Biomark Res. 2023;11(1):65. Published 2023 Jun 17. doi:10.1186/s40364-023-00501-9
- Tian X, Zhang R, Qin H, et al. Immunotherapy of B cell lymphoma with CD22-redirected CAR NK-92 cells. Cent Eur J Immunol. 2023;48(1):1-13. doi:10.5114/ceji.2023.126672
- Jan CI, Huang SW, Canoll P, et al. Targeting human leukocyte antigen G with chimeric antigen receptors of natural killer cells convert immunosuppression to ablate solid tumors. J Immunother Cancer. 2021;9(10):e003050. doi:10.1136/jitc-2021-003050
- Khawar MB, Gao G, Rafiq M, et al. Breaking down barriers: The potential of smarter CAR-engineered NK cells against solid tumors [published online ahead of print, 2023 Aug 11]. J Cell Biochem. 2023;10.1002/jcb.30460. doi:10.1002/jcb.30460
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.