Sponsored Content by MecmesinOct 18 2017
Specification
Founded in 1957, Kawasumi Laboratories Inc. was Japan’s first manufacturer of disposable plastic medical devices for blood transfusion and collection purposes.
Among their range of plasmapheresis and hemodialysis products, Kawasumi have developed a range of ‘painless’ arteriovenous (AV) dialysis needles.
Optimized to reduce tissue damage and therefore minimize pain, the design of the ‘constant-site cannulation’, otherwise known as ‘buttonhole technique’ needle aims to guard against the risk of harm to the fistula itself and prevent unintentional needlestick.
A unique bevel shape and silicone application technology are pivotal to attaining these patient comfort goals. Due to the “non sharp” point the ‘constant site cannulation’ device needs to be inserted at a pre-existing site tunnel.
Kawasumi Laboratories needed the capacity to evaluate alternate prototype needle designs, to test and therefore control the patient’s experienced discomfort level.
Solution

Mecmesin Asia worked alongside Kawasumi Laboratories to understand the essence of the insertion process, to be able to repeat the conditions necessary and allow replicable and accurate testing methods.
A custom fixture was designed to permit controlled adjustment of the insertion angle of the test sample, normally a standard specification of 15 degrees is used. To reproduce the toughness characteristics of human skin, a PVC sheet was placed over a lower fixture with sufficient clearance within the fixture to allow full passage of the needle through the membrane.
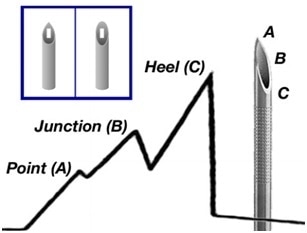
A standard graph plotting force against distance displays a typical shape with salient features representing the passage of elements of the tip of the needle through the membrane. Three peaks can be seen:
- At the point of initial penetration / puncture.
- As the needle aperture (junction) passes through the fistula there is an increase in force.
- As the heel is reached there is a final peak.
The force decreases and plateaus as the cannula’s constant bore body is inserted. The average measurements and peaks are calculated by the Emperor™ program during the cannula insertion segment.
Kawasumi’s R&D laboratory can use this practical data to minimize the exerted force by feeding back the results of the design comparisons, as well as using the data to further promote their products to their existing customers.
System
- Custom-designed fixture
- 50 N intelligent loadcell (ILC)
- MultiTest 2.5-i computer controlled system (Superseded by OmniTest 2.5 universal testing machine)
About Mecmesin
Established in 1977, Mecmesin is a global brand renowned worldwide for delivering reliable, affordable and innovative force, materials, and torque testing equipment for quality control.
Mecmesin is part of the Physical Properties Testers Group (PPT Group), a multi-national group of brands expert in the design and production of solutions for testing a range of physical properties including compression, light fastness, moisture, dry rate, water repellency, abrasion, flammability, texture, tensile and torque properties.
The PPT Group has regional offices in the UK, France, Germany, the USA, Thailand and China. The Mecmesin brand is also supported by a global network of distributors in more than 50 countries able to provide technical expertise and after-sales support to customers locally.
The focus of the brand has always been to provide high-quality test solutions, which are an affordable alternative to the many higher-priced systems available, enabling small and large businesses alike to undertake quality control checks on their products without compromising on precision and accuracy.
The rugged design of these systems means they can withstand tough factory conditions and perform tests at the point of production rather than having to use expensive laboratories to ensure consistent manufacture.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.