Introduction
In the 21st century, plastic medical tubing has become integral to effective healthcare. Tubing is used to deliver nutrients, blood, and gases to patients, as well as to perform minimally invasive procedures, for example, cardiac angioplasty, which over the past few years, have increased widely.
As a result, manufacturers have been driven to develop and launch more advanced tubing products in the market within shorter periods of time. The success of the device together with the safe, effective treatment of the patient can only be realized by paying close attention to both form and function during development, supported by high-quality and consistent production.
Applying force testing during the development of medical tubing offers an easy yet accurate means of qualifying the key mechanical characteristics of the device. Force testing helps optimize the functionality, performance, and compliance to international standards.
During production, force testing provides a fast and low-cost method to ensure consistent quality, thus preventing mistakes that could lead to lethal repercussions, and damaging financial and legal consequences for the brand.
This article analyzes four force testing procedures applied for plastic medical tubing products and examines the advantages and methodological considerations of each procedure.
Tension
When a medical tubing product is subjected to a simple tensile test, it can provide useful data to the developer when modeling the device’s predicted performance in situ. The tensile test can be used as a quality control approach in production to detect problems in advance during the extrusion process, as shown in Figure 1 and Figure 2.

Figure 1. Tensile testing a sample of thick medical tubing
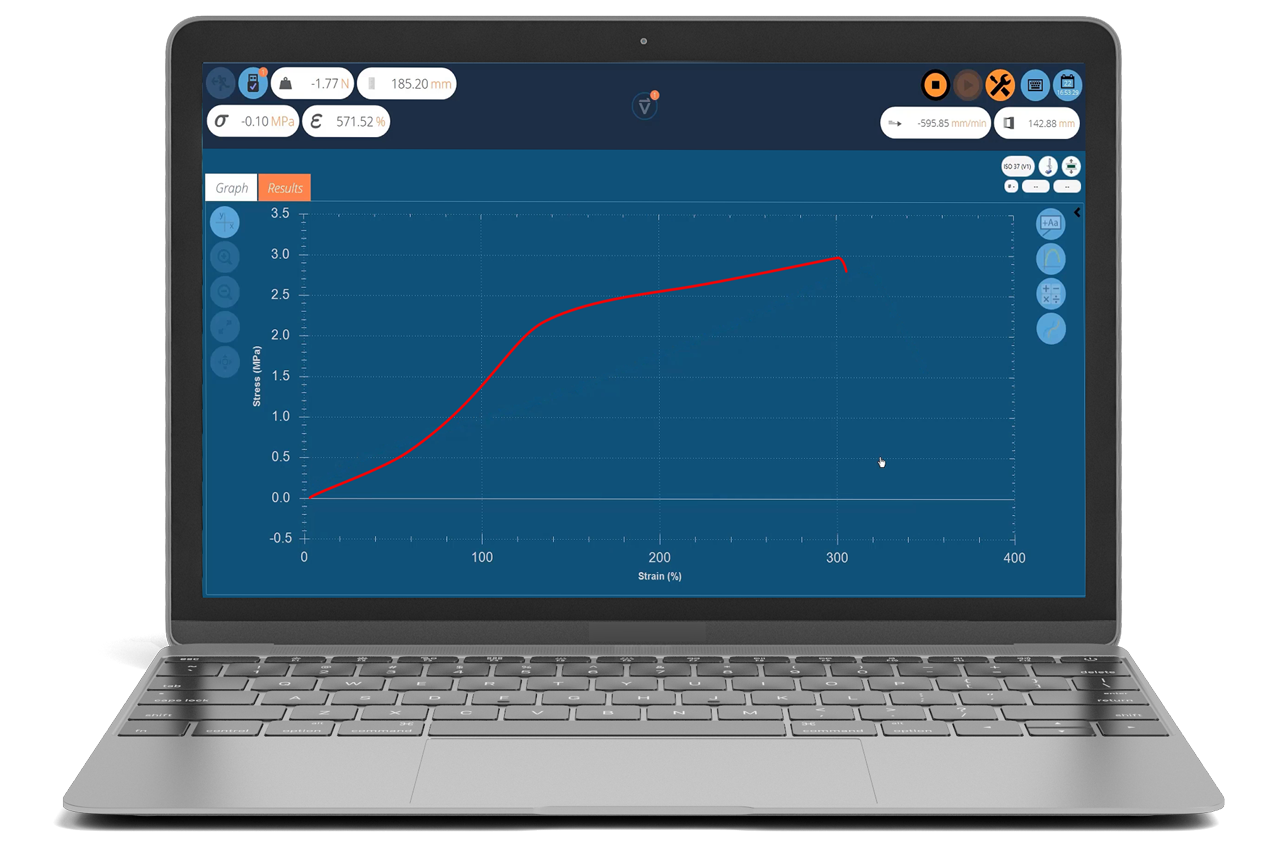 Figure 2. Resultant tensile stress-strain profile from a test of elastic tubing. The initial linear region, yield point (where the graph levels off) and eventual breaking point (where the graph ends) are clearly visible
Figure 2. Resultant tensile stress-strain profile from a test of elastic tubing. The initial linear region, yield point (where the graph levels off) and eventual breaking point (where the graph ends) are clearly visible
There is a growing demand to reduce the tubing’s wall thickness in order to:
- to reduce the trauma caused to fragile body tissues
- to provide additional internal space through which supplementary instruments may be introduced or fluids may flow
- to reduce mass production costs
Tensile tests are capable of assessing the effect of reductions in material usage in terms of the performance and strength of the tubing.
Young’s Modulus is a useful parameter that helps predict the performance of a material, which can be measured by mapping the elastic areas of the tubing’s stress-strain curve in the tensile test.
There are other standard measurements that include yield strength, the load at which the material deforms permanently and most importantly, tensile strength, the load at which point the tubing breaks completely. However, one aspect that would be of major interest is the tubing sample’s percentage elongation at breaking point, or at a predetermined force.
The test is carried out by wrapping each end of the tubing around a lower and upper circular bollard grip, which is mounted on an automated force testing system. A tensile load is applied slowly and steadily until observing the required parameter.
A simple console-controlled force testing system is more than adequate for routine tensile strength testing in the production floor. In the case of more complicated mapping of the tensile properties of the material, a computer-controlled system is generally employed to facilitate graphical display of the tensile profile of the tubing and to allow more advanced collection of results. To induce adequate elongation of larger samples, an extended crosshead travel may well be needed on the selected system.
Joint Strength
A wide range of techniques are used to join medical tubing to terminal connector components, with either reversible fittings such as barbed Luer fittings, as shown in figure 3, or permanent such as molding and bonding.

Figure 3. Disassembly strength tests of tubing from a winged infusion connector and other tube connector designs.
A major parameter in the performance and quality of the device is the tubing’s disassembly force from the terminal. The force should be high enough to prevent failure during use and guarantee a hermetic seal within the joint, but in a reversible fitting the assembly force should be low enough to guarantee accessibility in time-limited situations.
The disassembly test is carried out by holding the connector terminal in the upper fixture of an advanced automated force testing system. The sample can be effectively secured with a specialized mounting jig; this is followed by securing the tubing to a circular bollard grip from underneath.
As a result, the load is uniformly distributed across the tubing, and sample slippage during testing and early failure at the gripping face are prevented. Next, an axial tensile load needs to be applied at a steady rate until the tubing is fully displaced from the connector.
In order to evaluate the integrity of the seal inside a Luer joint, using a predetermined force, the tubing is pushed across the barbed taper. The tubing system is fed with compressed air and the joint is placed under water and visually examined for formation of bubbles for any sign of leakage.
Packaging
The packaging used for delivering the tubing products should be designed and manufactured with practically the same attention as given to the functionality and quality of the device itself. Packaging that is consistently produced and well-designed will not only protect the tubing but also retain sterility during storage and transportation, while remaining easily accessible on demand.
Tensile strength and elongation tests, similar to those described above, may be performed on packaging material in accordance with ISO EN 11607-1, Packaging for Terminally Sterilized Medical Devices, and EN 868-1, Packaging Materials and Systems for Medical Devices which are to be Sterilized, to guarantee conformance to minimum strength requirements. Likewise, pierce and rupture testing of film blister packs and airtight foil indicate the flexibility to applied forces that may be faced during the life cycle of the pack.
The ease with which the pack can be peeled open can be measured by the peel strength of the adhesive seals. The peel strength is generally determined using an automated force testing system equipped with a peel table fixture.
Lastly, the same system equipped with another specialized fixture that can be used to determine the CoF (Coefficient of Friction) of the packaging of the outer and inner surfaces. This makes it possible to optimize the form-fill-seal machinery settings during processing and shows how easily the device can be taken out of its packaging upon opening.
Trackability
Ever since the late 1980s, cardiac surgeons have been using percutaneous coronary intervention to clear arterial blockages in patients. Percutaneous coronary intervention is a highly efficient and minimally invasive surgical procedure, which considerably shortened trauma and recovery times when compared to the earlier surgical methods.
In this surgical procedure, a guide catheter is threaded through the complex network of arteries from the groin or arm to reach to the treatment site. This is followed by threading a thin, flexible metal guide wire via the catheter and extending beyond the blockage.
Another smaller catheter, with a balloon fixed to its tip, is passed over the guide wire and inflated in the narrowed section to remove the blockage, and if needed, a stent is placed in the vessel to keep its walls open.
Various parameters control how easily the guide catheter may be navigated via the arteries, and its trackability. These parameters include the lubricity of the external surface of the catheter and the forces applied by the tip of the catheter at critical points on the way to the affected area.
During the development of these devices, force testing enables designers to reduce the forces applied on fragile artery walls and thus lower the possibility of perforation.
Custom-engineered and sophisticated trackability testing systems are available that include a 2D or 3D model of the intricate network of arteries, as well as an interconnected system of delicate load cells that are capable of identifying the forces applied on the artificial vessel walls while feeding the guide catheter to the model.
However, less sophisticated equipment can also be used to perform more simple and basic tests. For instance, in early feasibility studies, simple tests that involve insertion and extraction of small catheter samples into tiny sections of synthetic artery may be conducted on a low-cost force testing system.
In a similar way, the frictional forces of the catheter and guide wire can be assessed when the two materials slide over one another in early feasibility studies.
The guide wire itself can be subjected to additional tensile testing, developers will be able to test the characteristics to ensure that it is extremely lightweight and maneuverable, and one that still has adequate strength to drive through heavily or severe calcified lesions inside the artery.
On the other hand, an automated force testing system with a unique fixture can be used to determine the coefficient of friction (CoF) of the external surface of the catheter, enabling lubricity comparisons of other prospective device materials.
However, these methods are not necessarily limited to coronary angioplasty, and analogous tests are well-suited for catheters that are specifically developed for other stent placement procedures, such as renal and peripheral angioplasty.
Effective Testing
This article has shown how a low-cost automated force testing system allows a wide range of key quality testing processes in the design and development of medical tubing products. From elongation and tensile strength tests, to joint integrity and lubricity, investment in the right instrumentation enables fast, simple and exact evaluation of the quality and functionality of tubing products, and represents an important element in the delivery of effective medical products in present fast-moving technological environment.
About Mecmesin
Established in 1977, Mecmesin is a global brand renowned worldwide for delivering reliable, affordable and innovative force, materials, and torque testing equipment for quality control.
Mecmesin is part of the Physical Properties Testers Group (PPT Group), a multi-national group of brands expert in the design and production of solutions for testing a range of physical properties including compression, light fastness, moisture, dry rate, water repellency, abrasion, flammability, texture, tensile and torque properties.
The PPT Group has regional offices in the UK, France, Germany, the USA, Thailand and China. The Mecmesin brand is also supported by a global network of distributors in more than 50 countries able to provide technical expertise and after-sales support to customers locally.
The focus of the brand has always been to provide high-quality test solutions, which are an affordable alternative to the many higher-priced systems available, enabling small and large businesses alike to undertake quality control checks on their products without compromising on precision and accuracy.
The rugged design of these systems means they can withstand tough factory conditions and perform tests at the point of production rather than having to use expensive laboratories to ensure consistent manufacture.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.