Sponsored Content by MecmesinOct 18 2017
Every year, billions of single-use needles as well as syringes are manufactured that range from surgical and hypodermic through to winged infusion sets and lancets. The medical device market is a continuously evolving due to the ubiquitous demand to reduce the large-scale production costs and the quick frequent introduction of new safety-oriented devices.

In order to ensure an effective development and production of medical devices, the mechanical properties of syringes and needles should be completely evaluated. The important physical characteristics of these devices can be quantified through force and torque testing, which provides a fast, simple, and relatively low-cost quantification method.
Manufacturers can also use these methods to rapidly detect and adjust for production errors which could endanger the comfort and safety of patients as well as the reputation of the brand in the long run.
Force and torque testing also assists designers to perfect the fitness-for-purpose and usability of their products, to reach an optimum balance between mechanical strength and material usage, while making sure that all new products adhere to international standards.
The following sections explores seven major areas where force and torque testing is applied for testing syringes and needles, keeping in mind the purpose, considerations, and benefits of each test, and wherever suitable, describes how these applications are generally carried out.
In any adhesive-sealed syringe packaging, the peel strength is generally tested to make sure that the device is sterile, secure, and easily accessible when required.
Needle Insertion and Penetration Forces
Needles should be cleanly inserted and extracted to improve patient’s comfort, so that minimum trauma is caused to the subcutaneous tissue and epidermis. Insufficiently sharp and malformed needle points can cause significant amount of pain during insertion, but this can be easily prevented with a consistent and well-designed device.
Point sharpness, the effectiveness of the beveled tip shape, and the frictional forces felt along the needle shaft are all characterized by determining the penetration and extraction forces of needles during the development and production stages. To perform this test, a motorized force testing system together with a unique lower fixture is used to hold a stretched foil of PU or PBC across a steel ring to replicate the skin.
The needle is placed on a low capacity and sensitive load cell and is brought down through the foil at a steady rate by the motorized crosshead travel of the test stand. A real-time, graphical force profile is provided by a computer-controlled system that can be examined for key target areas, including:
- The peak force at which point the tip of the needle initially penetrates the foil
- The force needed for the beveled edge to proceed through the hole, widening it
- The frictional force experienced as the shaft of the needle passes through the hole, after the bevel has been passing
Needle-to-hub Retention Strength
Various methods such as bonding, welding, molding, sealing, and mechanical interlocking are used to hold needles within their hub. There should be adequate retention strength of the assembly technique so as to prevent seal failure or disassembly in use.
Retention strength is generally tested by fitting the test system with an aluminum mounting block, and below this block the hub is located with the tip of the needle exposed vertically. With regard to smaller lancets and needles, a pin vice operated by a lever can be fitted onto the needle tip, followed by applying an axial tensile load at a continuous rate until the needle is fully displaced from the hub.
In the case of larger gauge needles, the sample may need to be held by a small wedge grip. This test is carried out in accordance with ISO 7864 international standard.
Surgical Needle Crimp Strength
Single-use surgical needles are generally supplied linked to a length of suture with a crimped end. This can be in the form of a drilled-end crimp, wherein a tiny aperture is made at the needle end, the suture is threaded within, and the needle is passed over it.
Single-use surgical needles may alternatively use a flange-type crimp, wherein the end of the needle is pressed into a semi-circular formation, with the suture placed within the groove and both ends of the semi-circle closed over it.
There should be adequate pull-off force when pulling the needle from the suture so that it does not come away while placing the sutures, but should be low enough so that surgeons can pull the needle from the suture as needed.
The pull-off test is carried out by holding the needle in a small wedge grip, and wrapping the suture material around a cable cam grip. This allows the load to spread uniformly, preventing slippage during the time of testing. Next, a tensile load is applied at a slow and steady pace until the suture is displaced from the crimp. The crimp retention strength is the highest tensile force experienced.
Needle Materials Testing
It is imperative that the steel needle itself is manufactured to a high standard of quality, and it is equally important that force testing plays a key role in productions, such as inline checks performed at steel formers and materials quality checks conducted at assemblers, as well as in development to detect ways to cut down material usage without affecting functionality.
Tensile Strength
This is a mechanical test to measure the needle’s material properties and identify areas of identified weakness. Pneumatic grips hold the steel needle at either end, exerting an adjustable pressure suitable for the needle gauge and preventing premature failure at the jaw face. To prevent collapse, small inserts can be threaded through the ends of the needle.
There should be lightly serrated faces in the pneumatic grips, so that the sample does not slip under loading. Then, an axial tensile load is gradually applied until a clean break takes place in a middle part of the needle, beyond the grips.
Three-point-bend
Needle stiffness is evaluated through the three-point-bend test and thus, the likelihood of permanent deformation may occur on insertion. In this test, a unique fixture is used comprising of a pair of adjustable lower support anvils on which the sample is placed, and one upper anvil positioned onto the automated testing machine’s motorized crosshead.
The top anvil slides down to a pre-determined height and causes a bend in the middle portion of the sample. Load analysis at a carrying vertical displacement enables measuring the needle’s Young’s Modulus, a valuable measure for defining its predicted behavior under usual loading in use.
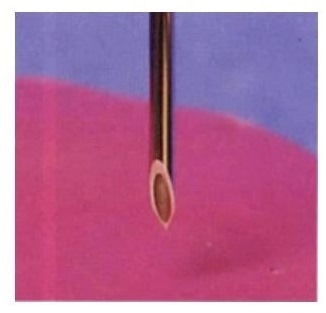
Penetration testing of hypodermic needles assesses point sharpness and geometry.

Needle hub pull-out tests commonly assess joint strength in development and production
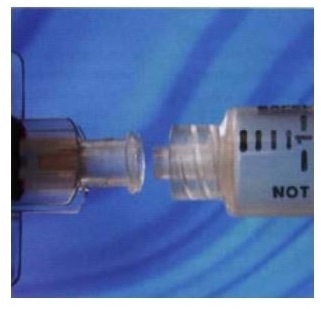
Threaded Luer lock connectors undergo torque testing to assess joint strength and ease of operation.
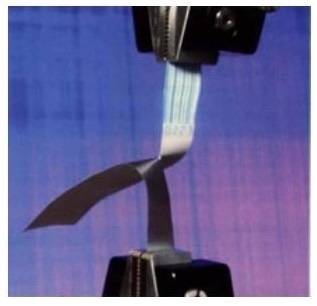
The tear strength of device packaging material is a critical parameter in assuring quality in production.
Torque assessment of Luer lock connectors
Needle hubs are capable of attaching to other devices including syringes through threaded Luer lock connectors or push/pull taper Luer.

The torque or force should be high enough to disassemble the two elements, preventing failure during operation and ensuring a hermetic seal in the connection, but low enough to allow fast and simple disassembly and engagement as needed by the healthcare professional.
With Luer locks, torque should be assessed on a low-level torque testing system with a low capacity (for example 1.5 Nm) torque transducer and a motorized base plate. The sample can be held in place through universal gripping pegs, but greater repeatability and accuracy are ensured through customized fixtures.
The general test includes tightening the Luer lock connection between a fluid-filled syringe and a needle to a pre-determined torque, and then visually examining the joint for any sign of leakage. This test has been illustrated in ISO 594 1/2 international standard.
Syringes
Syringes should be able to extract and deliver fluids in a smooth and controlled way. A syringe plunger that is too easy or hard to actuate or that shakes or stalls considerably on depression will not be able to perform consistently during aspiration or injection.
Aspiration force refers to the efforts required to draw a liquid into the syringe, and expression force refers to the efforts needed to eject liquid from the syringe.
These are determined by many variables, such as:
- the ‘fit’ of the plunger inside the syringe barrel
- the size of the needle/syringe aperture
- the viscosity of the liquid
- the density of the tissue substrate
During the development of the syringe, there should be an optimum balance these variables to ensure usability and consistency. ISO 7886 describes a technique which can be used to determine the aspiration and expression forces using a syringe that is half-filled with water and vertically placed onto a motorized force testing system.
The outlet of the syringe is linked to a reservoir (i.e., open to atmospheric pressure), and the water level is adjusted to match the fill level in the syringe chamber. An appropriate lower fixture is used to hold the piston from below.
When the system is initiated, it pulls down the syringe plunger at a steady rate, drawing the liquid into the chamber, and then records the aspiration force. The expression force is recorded by reversing the motor, and anomalous troughs and peaks in the force profile are tracked so that a smooth motion of expression and aspiration is rendered by the syringe.
Syringes are also subjected to the following common force tests:
- disassembly force testing of the piston’s rubber plunger
- chamber stress cracking evaluates compression resistance
- penetration testing of hypodermic needles evaluate point geometry and sharpness
- threaded Luer lock connectors go through torque testing to evaluate ease of operation and joint strength
Packaging
During syringe and needle packaging, care must be taken to ensure that the device is sterile, safe, and undamaged during storage and transportation, and at the same time, should be easily accessed when required.
The use of force and torque testing during design, processing, and production can significantly help in the development of easy and consistently reliable packaging. Some of the standard applications include:
- Pierce testing of foils and films employed on needle blister packs
- Peel strength of adhesive sealed syringe and needle packs to qualify ‘openability’
- Coefficient of friction assessment of packaging material to improve form-fill-seal machinery settings
- Tear testing, elongation, and tensile strength of flexible packaging material (in accordance with EN 869-1 and ISO 11607 standards)
Conclusion
Many useful torque and force testing applications are available throughout the lifecycle of syringe and needle devices, ranging from developing the needle, attaching it to its hub, and packaging it in the factory through to unpacking the needle, connecting it to a syringe, and injecting patients at the hospital.
These tests should be leveraged by producers and developers alike as they provide a fast, low-cost, and accurate method of evaluating the usability, quality, and compliance of their products to global standards.
Acknowledgements
Produced from materials that were originally produced by David Mercer, Mecmesin.
About Mecmesin
Established in 1977, Mecmesin is a global brand renowned worldwide for delivering reliable, affordable and innovative force, materials, and torque testing equipment for quality control.
Mecmesin is part of the Physical Properties Testers Group (PPT Group), a multi-national group of brands expert in the design and production of solutions for testing a range of physical properties including compression, light fastness, moisture, dry rate, water repellency, abrasion, flammability, texture, tensile and torque properties.
The PPT Group has regional offices in the UK, France, Germany, the USA, Thailand and China. The Mecmesin brand is also supported by a global network of distributors in more than 50 countries able to provide technical expertise and after-sales support to customers locally.
The focus of the brand has always been to provide high-quality test solutions, which are an affordable alternative to the many higher-priced systems available, enabling small and large businesses alike to undertake quality control checks on their products without compromising on precision and accuracy.
The rugged design of these systems means they can withstand tough factory conditions and perform tests at the point of production rather than having to use expensive laboratories to ensure consistent manufacture.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.