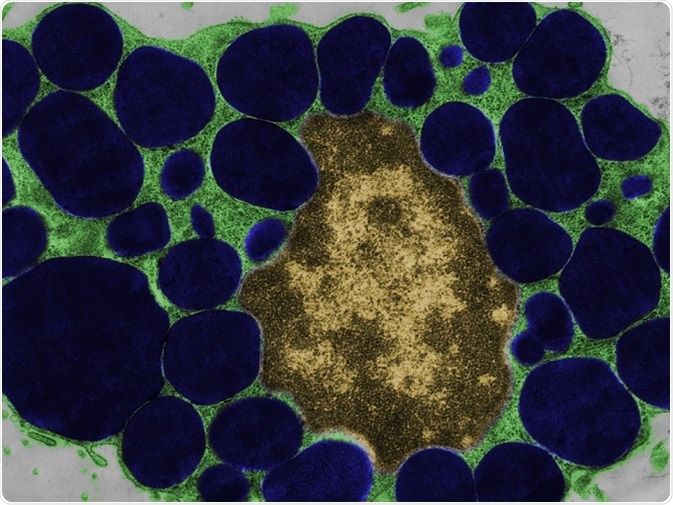
False colour transmission electron microscope (TEM) micrograph of a mast cell (mastocyte) with the cytoplasm (green) full of heparin granules (dark blue). © Jose Luis Calvo/shutterstock.com.
Heparin is a naturally occurring anticoagulant released from mast cells. It binds reversibly with anti-thrombin, which leads to increased inactivation of coagulation enzymes, whereby preventing blood clot formation. It is a mixture of sulphated glycosaminoglycan polymers.
Heparin is indicated for a range of disorders where blood thinning is desirable, such as cardiovascular disorders and intravascular coagulation, and for the prevention of post-operative deep venous thrombosis and pulmonary embolism. It is administered by intravenous injection or by intravenous infusion.
It is used either as unfractionated heparin that includes polysaccharides of varying molecular weight or as low molecular weight heparin. The average molecular weight of unfractionated heparin is 15 kD, compared with 4.5 kDa for low molecular weight heparin. Unfractionated heparin shows more specific binding than low molecular weight heparin but is associated with a higher risk of bleeding and higher risk of developing osteoporosis with long term use.
Despite many newer anticoagulants now being on the market, heparin and its lower molecular weight versions are the most used anticoagulant and antithrombotic drugs.
Sources of heparin for medical use
Currently, over half of the worldwide heparin demand is met by China. Heparin for medicinal use was traditionally obtained from the intestinal mucosa of cows and pigs. However, the outbreak of bovine spongiform encephalopathy (BSE) brought an end to the use of bovine-derived heparin in most countries. This considerably increased the demand for porcine-derived heparin.
The heparin market was subsequently further hit by the outbreak of a pig disease. Attempts to meet heparin demand despite reduced availability led to the adulteration of some heparin with the inexpensive semi-synthetic anticoagulant chondroitin sulphate.
This is more highly sulphated than heparin and caused severe side effects in many patients and even resulted in several deaths1. This incident highlighted the importance of ensuring the quality of products entering the market from the various heparin suppliers around the world.
Heparin-rich pig intestine harvested at slaughterhouses is transported to processing facilities where crude heparin is obtained. The crude heparin must then be further purified to the levels required for an active pharmaceutical ingredient. There are therefore many points at which contamination or adulteration could occur. To ensure patient safety it is thus critical that heparin quality is assessed during the manufacturing processes.
Quality control during heparin manufacture
Quality control of heparin has improved over the last decade with the introduction of nuclear magnetic resonance (NMR) spectroscopy and high-performance liquid chromatography (HPLC) testing. It is therefore unlikely that chondroitin sulphate contamination could go unnoticed at present. However, these monograph tests may not identify contamination with non-porcine sources of crude heparin.
The production of the final heparin product is strictly controlled, yet many manufacturers of medical grade heparin will not know the exact source of the crude heparin they use. Indeed, a batch of crude heparin may have been obtained from mucosa harvested at several different slaughter houses.
Heparin can be sulphated on one of several positions, which does not appear to modify the structure. However, addition of a sulphur atom at one of the possible sites would obscure the binding sites for anti-thrombin, preventing the anticoagulant activity of heparin.
The extent and pattern of sulphation is characteristic of the animal from which it was derived. It is therefore possible to characterize crude heparin by the sulphur distribution and ensure that it meets the normal range values.
Despite various analytical techniques being employed to assess medical grade heparin, few of them have been used to determine the structure and composition of crude heparin. In fact, the average composition of crude heparin in the global marketplace had not been determined. Researchers have now started to address this.
NMR spectroscopy and chemometrics for structural analysis
In a recent study, NMR spectroscopy and chemometrics were used to characterize 88 crude heparin samples collected between 2010 and 2015 from 13 different manufacturers2. The structural features of heparin were differentiated according to their source using a multivariate analysis of the NMR spectra.
In addition, the quantitative composition of crude heparin was determined using heteronuclear single quantum coherence spectroscopy. Purity levels of the samples were assessed using strong anion exchange HPLC. Spectroscopy measurements were obtained using a Bruker AVANCE III 600 MHz spectrometer or Bruker AVANCE III HD 500 MHz spectrometer. The spectra were processed and integrated using Bruker Topsin software.
The data showed that the crude heparins tested varied in glycosaminoglycan composition, amount of DNA/RNA, and other impurities. This indicates that different levels of purification had been achieved by different manufacturers, which is consistent with the known usage of different purification processes.
The NMR Spectroscopy imaging methodology used is thus a suitable way to distinguish between crude heparins originating from different animals or organs and manufactured using different processes.
References
- Guerrini M, et al. Oversulfated chondroitinsulfate is a contaminant in heparin associated with adverse clinical events. Nat. Biotechnol. 2008;26:669–675.
- Mauri L, et al. Combining NMR Spectroscopy and Chemometrics to Monitor Structural Features of Crude Heparin. Molecules 2017;22:1146.
About Bruker BioSpin - NMR, EPR and Imaging

Bruker BioSpin offers the world's most comprehensive range of NMR and EPR spectroscopy and preclinical research tools. Bruker BioSpin develops, manufactures and supplies technology to research establishments, commercial enterprises and multi-national corporations across countless industries and fields of expertise.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.