Reproducibility and stability are crucial for highly meticulous laboratories like those of pharmaceutical companies, regardless of the technique. Consistent results must be gathered using identical protocols. Bruker’s MRI instruments provide researchers with the reliability needed by meeting this challenge.
Pre-Optimized Protocols and Scan Programs
Bruker’s MRI instruments have delivered, with pre-optimized protocols which have been tested on small rodents and categorized according to animal, anatomical region, and application for decades (Figure 1). Employing these stored protocols makes sure that scan parameters are identical from one scan to the next.
Scan to scan importation of parameters guarantees consistency throughout studies and subjects if parameters are adapted. ParaVision® 6 marked the introduction of entire scan programs and automatic report generation. These study reports maintain optimum scientific standards and can be customized to contain representative images from each scan, ensuring that every scan parameter is recorded for future reference (Figure 1).
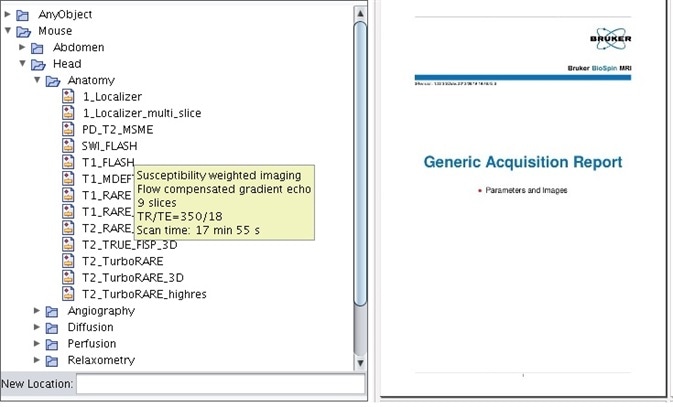
Figure 1. Pre-optimized protocols for mice and rats, categorized via anatomical region and application, come standard with the instrument. All parameters and representative images can be saved in the study report.

Figure 2. The CASL workflow package with its pre-prepared, complete scan program, examination guide, and integrated reconstruction with automatic measurement of inversion efficiency and output of quantitative CBF maps provides consistently reliable operator independent data.
The CASL Workflow Package was developed in cooperation with Emmanuel Barbier, Grenoble Institute of Neuroscience. Courtesy: E. Barbier, L. Hirschler, J. Warnking, Grenoble Institute of Neuroscience
Examination Guide with Quantitative Results
ParaVision 360 introduces the workflow package for Arterial Spin Labeling (ASL) to explore the scan programs even more (Figure 2). This package was developed in an industrial-academic cooperation with the Grenoble Institute of Neuroscience and gives optimal results when utilized in combination with a dedicated Arterial Spin Labeling coil1.
It has an examination guide which aids the user throughout scan preparation, a full pre-prepared scan program, and an integrated reconstruction with automatic measurement of inversion efficiency and output of quantitative Cerebral Blood Flow (CBF) maps.
Superior Image Quality for Streamlined Evaluation
Based on Bruker’s patented IntraGate, ParaVision 360 also introduced the IntraGateUTE technique. It is also incorporated into IntraGateFLASH, a technique which has been championed by ParaVision users for years. IntraGate based techniques are self-gated, needing no external triggering devices or electrodes. This improves animal welfare and also saves valuable set-up time.
Reconstruction of respiratory, cardiac, or respiratory and cardiac cines can be carried out and the number of cine frames can be specified after the acquisition is completed. For optimum cardiac imaging quality, IntraGateUTE combines the advantages of IntraGate with those of the ultra-short echo time, radial readout UTE method.
It allows full murine heart cine coverage in less than 15 minutes and can be accelerated up to 8-fold over standard techniques (Figure 3). Due to its significantly decreased turbulence artifacts, streamlined evaluation with automatic algorithms is permitted.
Longitudinal Consistency
As proven by a research group at the University of Ulm, this excellent cardiac imaging quality is proven to be stable over time. In their research utilizing the IntraGateFLASH technique, the end-diastolic volume (EDV), endsystolic volume (ESV), ejection fraction (EF), stroke volume (SV), and left ventricular mass (LVM) of five female wildtype C57/B6 mice were measured on five days over the course of a year2.
No significant differences of ESV, EF, EDV, or SV were noted (Figure 4). As was to be anticipated with increasing age, the LVM grew significantly. This investigation shows the longitudinal reproducibility of the technique and confirms that the reliable software and hardware allow longitudinal studies to be performed uninterrupted.
Multi-Site Reproducibility
This reproducibility is also achievable from site to site and instrument to instrument. In their research article titled “Accuracy, repeatability, and reproducibility of longitudinal relaxation rate in 12 small-animal MRI systems”3 11 sites measured T1 in identical phantoms in order to access reproducibility on preclinical imaging systems (Figure 5).
The 11 sites were located within the United States of America, Sweden, the United Kingdom, Germany, and the Netherlands, headed by Bioxydyn Ltd, Manchester, United Kingdom. They utilized 12 MRI systems at field strengths ranging between 3 T to 11.7 T, namely 3 T, 4.7 T, 7 T, 9.4 T, and 11.7 T. The sites were permitted to employ preclinical MRI instruments from a manufacturer of their choosing.
Every site decided independently to employ a PharmaScan or Bruker BioSpec instrument (three of the 12 instruments contained magnets from the former Magnex, Varian, or Agilent companies) run on ParaVision software (versions 5.1 to 6.0.1 were utilized).
Each site acquired five phantoms, which were identical from site to site and were asked to calculate T1 in each of the phantoms using the RARE sequence (standard T1map_RARE protocol) and with a 2-parameter fit in ParaVision in three regions of interest. The measurement was repeated on a second day within a three week period, giving a total of 360 measurements.
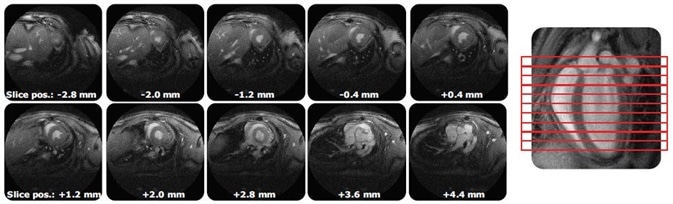
Figure 3. IntraGateUTE with its full murine heart cine coverage with significantly reduced flow artifacts leads to streamlined evaluation.
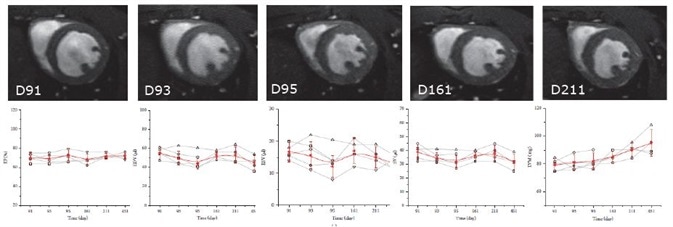
Figure 4. IntraGateFLASH measurement of cardiac parameters over course the of one year. Top: IntraGateFLASH images at days 91, 93, 95, 161, and 211. Bottom from left to right: ejection fraction (EF), end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), left ventricular mass (LVM) versus time.
Courtesy: V. Rasche, Core Facility Small Animal Imaging, Ulm University, Ulm, Germany.
Reference: Z. Zuo, et al., BioMed Research International, 2017
They discovered a Coefficient of Variation (CoV) of 1.43% between sites, which is significantly better than CoVs of 5.5% - 8.2% established in similar studies carried out with clinical systems.
This further reinforces the feasibility of T1 as a biomarker in studies of, for example, oncology, neurology, and cardiac and liver diseases, in addition to diseases such as multiple sclerosis, or myocardial fibrosis where minute alterations in T1 may prove to be clinically significant. Furthermore, it clearly shows the exceptionally reliable reproducibility of Bruker MRI instruments.
Functional Imaging Robustness
Yet, this reproducibility is not just limited to phantom studies, but can be seen even in such challenging examinations like fMRI. In their 2018 ISMRM paper titled “Multi-centre resting-state fMRI comparison reveals common functional networks in the mouse brain”4, resting state fMRI data gathered from C57B6/J mice on Bruker MRI instruments found at 12 different sites were compared.
These sites were within Canada, China, Australia, Singapore, Italy, Germany, the United Kingdom, Belgium, France, and Switzerland. The data was gathered under a number of conditions concerning coil type (room temperature or MRI CryoProbe), field strength (4.7 T, 7.0 T, or 9.4 T), and anesthesia type (medetomidine, halothane, isoflurane, or a combination of medetomidine and isoflurane).
Functional Connectivity (FC) values were established using two Region of Interests (ROI), in the contralateral and posterior cingulate cortex, based on seed points found at anterior S1 and the anterior cingulate, respectively. Significant contralateral FC for the anterior S1 seed was noted in between 60-80% of the datasets, while FC reproducibility for to the anterior cingulate seed was from 40-60%.
On the variations in setup mentioned above, the dependence of the FC data was examined and a positive correlation was discovered between FC for the anterior S1 and SNR, which in turn had a positive correlation with coil type and field strength (MRI CryoProbe yielding higher SNR). In both ROIs, anesthesia type was discovered to have an effect on FC.
The data from global imaging sites exhibit robustness of fMRI and establish that taking study setup into consideration, data gathered within international collaborations can be compared reliably.
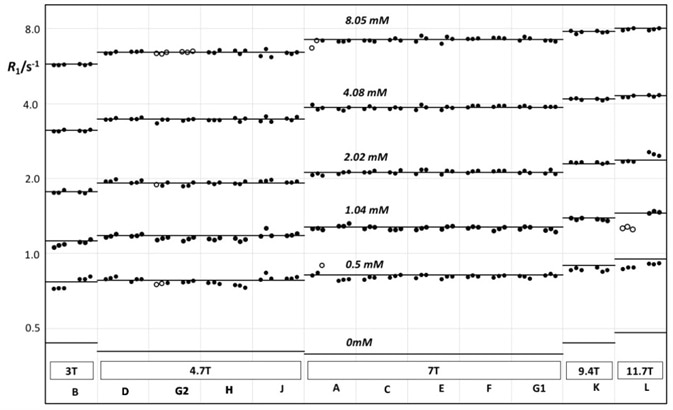
Figure 5. R1 vs. field strength, measured on centrally prepared phantoms on 12 different Bruker MRI instruments at 11 different sites.
Reference: J.C. Waterton, et al., Accuracy, repeatability, and reproducibility of R1 in 12 small-animal MRI systems, BCISMRM 2018, PO19.
Courtesy: J.C. Waterton CSci FRSC(UK), Tristan Consortium
Conclusion
The consistency of the data gathered using Bruker MRI instruments which are run on ParaVision, enables researchers to trust that their instruments will perform over time and to be confident of their results when comparing them with international reports, enabling them to perform longitudinal studies reliably and uninterrupted.
The optimum quality of the images allows evaluation using automatic algorithms, eliminating variation of results due to user dependencies. This quality is reliably and easily repeated thanks to pre-optimized scan programs and protocols, meeting the standards of even the most stringent laboratories.
References
- Hirschler L, Debacker CS, Voiron J, K.hler S, Warnking JM, Barbier EL (2017) Interpulse phase corrections for unbalanced pseudocontinuous arterial spin labeling at high magnetic field. Magnetic Resonance in Medicine 79(3)1314-1324
- Zuo Z, Subgang A, Abaei A, Rottbauer W, Stiller D, Ma G, Rasche V (2017) Assessment of Longitudinal Reproducibility of Mice LV Function Parameters at 11.7 T Derived from Self-Gated CINE MRI. BioMed Research International 2017
- Waterton JC, Hines CDG, Hockings PD, Laitinen I, Ziemian S, Campbell S, Gottschalk M, Green C, Haase M, Hassemer K, Juretschke HP, Koehler S, Lloyd W, Luo Y, Persson IM, O’Connor JPB, Olsson LE, Pindoria K, Schneider JE, Sourbron S, Steinmann D, Strobel K, Tadimalla S, Teh I, Veltien A, Zhang X, Schütz G (2019) Repeatability, and reproducibility of longitudinal relaxation rate in 12 small-animal MRI systems. Magnetic Resonance Imaging, https://doi.org/10.1016/j.mri.2019.03.008
- Grandjean J, Canella C, Anckaerts C, Ayrancı G, Coletta L, Gallino D, Gass N, Hübner N, Kreitz S, Mechling AE, Strobelt S, Wu T, Wank I, Chakravarty M, Chang WT, von Elverfeldt D, Harsan LA, Hess A, Keliris G, Rudin M, Sartorius A, Jiang T, Van der Linden A, Verhoye M, Weber-Fahr W, Wenderoth N, Zerbi V, Gozzi A (2018) Multi-centre resting-state fMRI comparison reveals common functional networks in the mouse brain. ISMRM (2018) 1108
About Bruker BioSpin - NMR, EPR and Imaging

Bruker BioSpin offers the world's most comprehensive range of NMR and EPR spectroscopy and preclinical research tools. Bruker BioSpin develops, manufactures and supplies technology to research establishments, commercial enterprises and multi-national corporations across countless industries and fields of expertise.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.