Sponsored Content by PolytecJan 14 2020
To be effective in treating respiratory illnesses, liquid drugs must be utilized in an aerosol with an effective and defined drop size distribution for inhaled medications. To meet these intense demands, the aerosol is created by the nebulizer system.
Compact laser vibrometers (CLVs) are used by one of the leading German inhalation system manufacturers, Pari GmbH, by measuring sophisticated membrane vibration characteristics to ensure the essential quality of the aerosol generator, which is the crucial part of the nebulizer system.
Performance of a Nebulizer System
The dose of the liquid drug intended for inhalation is fed into the reservoir via the medication cap. Components of the aerosol generation system are displayed in figure 1. An aerosol generation comes from the liquid in the medication reservoir within the nebulizer, with the assistance of a perforated membrane, whereby piezo elements actuate the membrane at ultrasonic frequencies. The resulting change in pressure behind the membrane nebulizes it by forcing the fluid through the perforations in the membrane (figure 2).
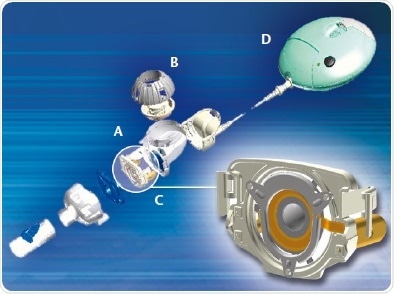
Figure 1. Design of an aerosol generation system. A, Medication reservoir; B, Medication cap; C, Nebulizer; D, Control unit.

Figure 2. Nebulizer with medication reservoir and medication cap as well as a magnified view of the nebulizer membrane showing micro-machined perforations.
Vibration Test on Aerosol Generators
A PSV scanning vibrometer was used to accurately analyze the deflection shape of the membrane throughout product development at Pari GmbH. Based on these results as well as others, the optimal membrane structure and the most appropriate resonant frequencies for optimal inhalation were defined. These comprehensive tests could characterize the vibrational behavior of the membrane of the nebulizer.
The CLV comprises a control unit and a compact sensor head connected by a flexible fiber-optic cable. Figure 3 displays where the semi-automatic test station was developed and installed in production for quality control. The diagnosis software QuickCheck and a compact single-point laser vibrometer are included in the test stand for noise and acoustics. The vibration features of each aerosol generator membrane are measured without contact using the CLV with a bandwidth of 350 kHz during production and the frequency spectrum is thus calculated (see figure 4).
Figure 4 also shows that certain frequency ranges are automatically analyzed for critical characteristics in the spectrum with Polytec’s QuickCheck production testing software, whereby each area is examined for the shape of their envelope curve, the presence or absence of peaks and peak-splitting.
The signal-to-noise ratio is calculated as an evaluation criterion for measurement quality. On behalf of the backscattered light for each sample, the laser spot location is evaluated, ensuring that the location is right between the perforations of the membrane. Figure 5 illustrates that, until the full laser intensity is restored, the sample point is automatically shifted by a few micrometers using a beam deflector if laser intensity is too low.

Figure 3. Complete test station for 100% quality control of nebulizer membranes.
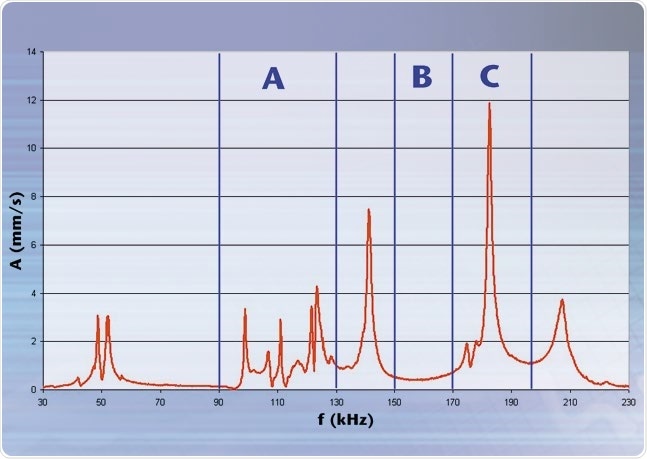
Figure 4. Typical spectrum of a membrane: A Envelope Assessment B Void area (no peak allowed) C Area in which there may only be one peak.

Figure 5. The semi-automatic test stand with integrated CLV Compact Laser Vibrometer and beam deflection unit.
Summary
The combination of diagnosis software such as QuickCheck and non-contact vibration measurements with a compact laser vibrometer allows the fast, quality testing and analysis for aerosol generator membranes. Within the first six months of operation, the semi-automatic test station and non-contact measurement system have completed over 10,000 inspections and, during this period, the analysis algorithm has been continuously improved to further minimize superfluous false rejects, guaranteeing the precise, reproducible delivery medication and the continuous quality of aerosol generation systems.
About Polytec

For over 50 years Polytec has provided high-technology, laser-based measurement solutions to researchers and engineers. Our commitment is to provide the most precise and reliable optical instruments and sensors available for non-contact measurement, setting Polytec apart from the competition as the gold standard in the design and manufacture of vibrometer and velocimeter systems. Our innovations answer many pressing manufacturing and engineering challenges.
Polytec was founded in 1967 to distribute commercial laser technology to industrial and research markets. Building on the company’s early success, Polytec began to develop and manufacture innovative laser-based test and measurement instruments in the 1970’s. These products have become known around the world as the gold standard in non-contact, laser-based measurement of vibration, speed and length.
Advanced product development remains a core strategic activity at Polytec with new electro-optical systems being designed for applications such as analytical process measurement, noise analysis, and factory automation.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.