Key Issues
- Synchronous in-process monitoring of metabolites, cell attributes, and nutrients with one probe.
- Process understanding is enhanced through Raman quantification
- Real-time, Raman-based model transferability and process control.
- Quantitative and specific process knowledge
- Adheres to the U.S. FDA Quality by Design (QbD) and Process Analytical Technology (PAT) initiatives.
Introduction
Critical process parameters (CPPs) in mammalian cell-based bioprocesses are biochemical properties (viability and cell count), chemical properties (waste concentrations, nutrients, and pH), and physical parameters (agitation rate, temperature, and dissolved oxygen(DO)).
It is crucial that these CPPs are carefully managed to maintain process quality and adhere to the strict specifications on product variability in cGMP manufacturing outlined by the U.S. Food and Drug Administration (FDA).
In situ monitoring of CPPs has normally been restricted to DO, pH, pressure, and temperature as sensors are available to quantify these parameters.
Biochemical and chemical features are normally quantified at-line or off-line. The nature of at-line or off-line analyses is inherently time-consuming and is incompatible with real time process control.
Raman advantages
Biochemical and chemical data can be easily gathered due to innovations in Raman spectroscopy. Raman has been commonly used for non-destructive, non-invasive process monitoring in many sectors, for example enabling real-time process control in biopharmaceuticals.1,2
The benefits of Raman for bioprocess control and monitoring are the simultaneous quantification of several biochemical and chemical parameters, the enabling of cross model scalability, and the ability to collect highly specific data across the spectrum.
Raman offers a non-destructive, non-invasive assessment with no requirement for additional reagents or sampling. Scientific and engineering developments in control and measurement have advanced to provide innovative process control strategies.
Real-time feed control based on Raman spectroscopy is now commonly utilized in biopharmaceutical organizations, from laboratories to cGMP. The investigation described in this article shows effective process development and transfer from benchtop to manufacturing.
Experimental
Kaiser Raman analyzers were utilized in situ to measure the following CPPs simultaneously: viable cell density (VCD), total cell density (TCD), osmolality, ammonium, lactate, glutamate, and glucose in a fed-batch bioprocess utilizing a Chinese hamster-ovary (CHO) cell line.
In situ examinations were carried out on bioreactors at the 3 L process development scale, 200 L pilot scale, and the 2000 L manufacturing scale. The spectral information was matched with off-line reference data employing both partial least-squares (PLS) regression and spectral preprocessing.
A Kaiser Raman multichannel analyzer (λ=785 nm) was the Raman instrument employed in this investigation, featuring stainless steel immersion probes (bIO-PRO pilot for production-scale runs and bIO-LAB-220 for benchtop) (Figure 1).

Figure 1. Real-time, in situ, probes optimized for bioprocess conditions enable measurements in laboratory (left) and manufacturing (right), with easy transfer of the analytical model. Image Credit: Kaiser Optical Systems, Inc.
Results
Calibration models were produced for each individual CPP being investigated: TCD, VCD, lactate, glucose, ammonium, osmolality, and glutamate.
PLS model predictions were created from batches at the process development (3 L), pilot (200 L), and manufacturing (2000 L) scales and were utilized to estimate the results from a manufacturing scale batch. Figure 2 demonstrates how Raman predicted results are highly similar to quantified values.
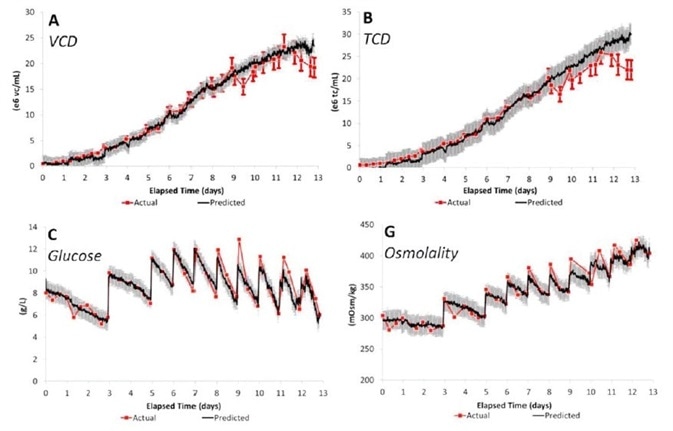
Figure 2. PLS model prediction results for major CPPs. Calibration data from batches at all three scales (3, 200, and 2000 L) were used to predict results from a batch at the 2000 L manufacturing scale. Image Credit: Reprinted with permission from Ref. 1. © 2014 American Institute of Chemical Engineers.
Conclusions
The investigation results show that Raman spectroscopy can be utilized to gather quantitative data on several CPPs at the same time. A bioprocess compatible Raman analyzer can quantify several parameters and real-time quantitative data on several nutrients in situ at the same time.
The investigation additionally shows that Kaiser Raman generates dependable results at manufacturing, pilot, and benchtop scales and that robust process models for application in the manufacturing industry can be created using these results.
The abundance of bioprocess data gathered through Raman spectroscopy creates exciting new avenues of bioprocess understanding, facilitates QbD, and offers real-time control of the bioprocess to enhance cell viability and titer.
References and Further reading
- Berry et al. Cross-scale predictive modeling of CHO cell culture growth and metabolites using Raman spectroscopy and multivariate analysis. Biotechn. Prog. 2015; 31(2), 566–577.
- Abu-Absi et al. Real time monitoring of multiple parameters in mammalian cell culture bioreactors using an in-line Raman spectroscopy probe [Communication to the editor]. Biotechnology and Bioengineering 2010; 108(5), 1215–1221.
About Kaiser Optical Systems, Inc.
Kaiser Optical Systems, Inc. (Kaiser), an Endress+Hauser company, is the global leader in Raman spectroscopic instrumentation for laboratory, process, and manufacturing environments. Our solutions harness the powerful analytical information of Raman Spectroscopy to help our customers understand, measure, and control their chemistries.
As a trusted partner in Raman for over 30 years, Kaiser has a long history in production, including GMP manufacturing, with many proven successes. Our unmatched expertise, high quality solutions, and exceptional customer service sets Kaiser far above any other Raman option in the marketplace. Kaiser Raman technology is currently used throughout the chemical, food and beverage, oil and gas, pharmaceutical, and biopharmaceutical industries to optimize process efficiency and deliver quality products. Kaiser’s manufacturing and headquarters facility is in Ann Arbor, Michigan.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.