Key issues
- Real-time, in-process understanding
- Quantitative monitoring of excipient and protein concentration using a single probe in ultrafiltration/diafiltration operations
- Testing and release of formulation buffers
- Real-time process and product quality assurance
- Kaiser Raman is a proven Process Analytical Technology (PAT)
Figure 1. Raman provides quantification on multiple components during downstream operations using a single probe. Image Credit: Kaiser Optical Systems, Inc.
Introduction
The 2004 process analytical technology (PAT) framework released by the US Food & Drug Administration (FDA) highlighted a shift from conventional quality assurance methods. The framework suggested a move from tested-in quality after drug products were created, to building in quality throughout the production process with “continuous real-time quality assurance.”
Also included in the PAT framework were the principles of implementation of “Quality by Design” (QbD), which promotes quality assurance through understanding and controlling the critical process parameters that affect a biologic product’s critical quality attributes. To guarantee high-quality biological products, the understanding of this process must be shared to downstream bioprocesses too.
Rapid and non-destructive spectroscopic methods provide options for the monitoring and control of downstream bioprocess operations, including monitoring purification cycles in-situ, identifying buffers, the aggregation of proteins1, identifying products2, and quality control.
Raman advantages
Research is currently concerned with attaining a fuller understanding of biologic downstream steps. This can be achieved by quantitatively measuring key analytes, such as amino acids, sugars, and proteins.
Technology similar to this has been utilized in order to trend nutrient and metabolite concentrations both in-situ and in real time during upstream bioprocessing stages3. Raman spectroscopy offers unique advantages in biotechnology QbD applications as it facilitates fast, non-destructive monitoring and control.
This article looks into the development of new bioprocess analytics and its application in relation to the goals set out by the principles of QbD.
As a technique in this area, Raman is user-friendly and offers flexible sampling benefits, such as the remote location of the analyzer, non-contact sampling optics, and compatibility with flow cells.
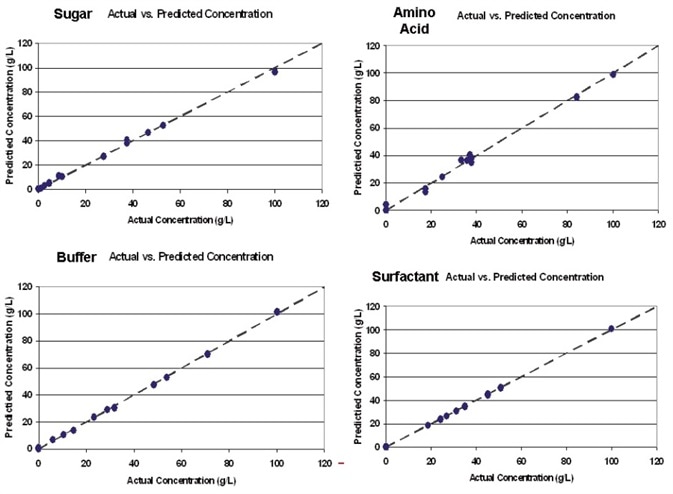
Figure 2. Kaiser Raman spectroscopy provides quantification on process components similar to offline laboratory measurement, but in real-time and in-process. Image Credit: Kaiser Optical Systems, Inc.
Experimental
Two downstream bioprocessing applications were studied at a leading biotech company. These included the testing and release of formulation buffers and monitoring excipients in ultrafiltration/diafiltration (UF/DF) operations.
To carry out these investigations, a Raman Rxn2™ analyzer equipped with a 785- nm Invictus™ laser and a fiber-coupled probe, which was fitted with an in-situ immersion optic was used.
To collect Raman spectra, the probe was inserted directly into each prepared sample, and for every sample, Raman data was collected for 30 seconds.
For the first application, mixtures of four components comprising amino acid, buffer A, sugar, and surfactant, of a formulation buffer were prepared according to the design of experiments (DoE). Protein was added to a three-component buffer system in the range of 0-100 mg/mL according to a DoE, comprising amino acid, buffer B, and sugar, to carry out the UF/DF application. Partial least squares (PLS) multivariate calibration models were used in order to correlate concentrations to the collected Raman spectra.
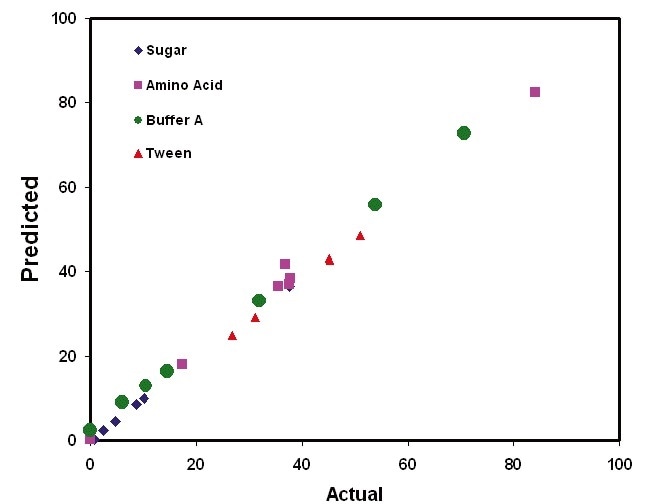
Figure 3. Kaiser Raman analysis provides quantification on multiple process components during UF/DF operations. Image Credit: Kaiser Optical Systems, Inc.
Results
Figure 2 illustrates the calibration models that were created for each buffer constituent. These models went on to be used to predict the concentrations of sample mixtures in a new sample set. Figure 3 shows the results of the predictions generated from the UF/ DF application. These results demonstrate the ability to quantify protein in addition to excipients.
Summary
Kaiser Raman technology offers reliable testing and methods of release for buffers, and for controlling UF/DF operations. As a technique, Raman gives users a simple and accurate method of analysis for aqueous-based systems and offers chemical specificity for both excipients and drug products. The results detailed in this article show the efficacy of Raman technology to support a QbD manufacturing environment that requires real-time, in-situ monitoring of downstream bioprocesses.
References
- Mungikar, A. et al. Am. Pharm. Rev., November/December 2010, 78–83.
- Wen, Z.-Q. et al. Am. Pharm. Rev., May/June 2010, 46–53.
- Abu-Absi, N. R. et. al. Biotech. Bioengin. 2011, 108 (5), 1215–1221.
About Kaiser Optical Systems, Inc.
Kaiser Optical Systems, Inc. (Kaiser), an Endress+Hauser company, is the global leader in Raman spectroscopic instrumentation for laboratory, process, and manufacturing environments. Our solutions harness the powerful analytical information of Raman Spectroscopy to help our customers understand, measure, and control their chemistries.
As a trusted partner in Raman for over 30 years, Kaiser has a long history in production, including GMP manufacturing, with many proven successes. Our unmatched expertise, high quality solutions, and exceptional customer service sets Kaiser far above any other Raman option in the marketplace. Kaiser Raman technology is currently used throughout the chemical, food and beverage, oil and gas, pharmaceutical, and biopharmaceutical industries to optimize process efficiency and deliver quality products. Kaiser’s manufacturing and headquarters facility is in Ann Arbor, Michigan.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.