In the lab, various phases of drug development occur in life sciences as new drugs and therapies are discovered and developed through extensive research. Labs are then used to ensure quality control monitoring as potential drugs move through process development on a pilot scale, followed by non-GMP and then GMP manufacturing.
Analytical development happens extensively throughout the lab-scale research phase. Lab-scale process development takes place once a potential new medicine has advanced through research and demonstrated performance.
Process development on a small scale, where genuine production processes can be built along with the creation of standard operating procedures (SOPs), is the next step.
The transmission of procedures and methods must adhere closely to the original lab guidelines at every stage. The planned progression of this transitional process from early lab activities to full-fledged manufacturing is aided by the reliability of analytical measures and technologies.
This article explores the use of process and analytical tools to speed up the transition of pharmaceuticals from the lab to mass production, particularly by substituting real-time monitoring for lab-based testing.
The role of lab testing
Labs play a crucial role in quality control during the entire drug and therapy production process, discharging products at every stage of the procedure to comply with FDA regulations. Drugs cannot move on to the next stage of manufacture without lab approval.

Figure 1. Lab measurements have been used for decades but can present problems. Image Credit: Endress+Hauser
Thorough lab analysis and process validation are required for these signoffs. Measurements of pH, conductivity, dissolved oxygen, optical density, ultraviolet (UV) absorbance, and other variables are essential to processes in the life sciences (Figure 1). There may be problems when these metrics are tested in the lab.
Methods used for sampling, improper sample handling, and differences between outcomes of lab analysis and real-time observations can all result in inconsistent results. A sample taken from the procedure may not have the same characteristics as the process media in terms of temperature, degree of mixing, and other elements.
Even when everyone is adhering to SOPs, further variation may still happen as a result of incorrect sample handling or merely discrepancies among specialists.
There will be a delay between the extraction procedure and test findings even if the sample coincides with the process medium when it arrives at the lab. Due to this gap, it is generally difficult to apply closed-loop control to a manufacturing process, and open-loop changes may be challenging to accomplish.
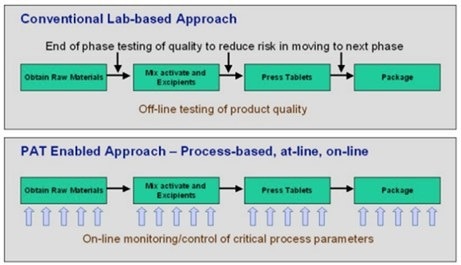
Figure 2. A PAT-enabled approach requires replacement of lab-based offline testing of product quality and other parameters with online monitoring. Image Credit: Endress+Hauser
Problems emerge in both situations as it takes time for the effects of adjustments made to enhance sample attributes to become apparent. Shifting measurements online to get real-time data can solve many properties’ problems with lab testing.
Lab-to-process digital liquid analysis
The manufacturing processes for life sciences are being streamlined nowadays owing to the introduction of process analytical technology (PAT), which makes more of the analytical techniques used in laboratories available for use in production processes.
These advancements are made feasible by new tools and analyzers that can do online measurements for some qualities that previously required laboratory testing (Figure 2).
Bringing lab measurements into the workflow to expedite release, enhance time to market, and reduce costs while preserving quality is one component of PAT. For PAT to be applied correctly and securely, this strategy necessitates uniformity between production measurements and lab analytical procedures (Figure 3).
Lab-to-process implementation calls for the transmission of off-line measurements utilizing standardized technology in the lab and in the process.
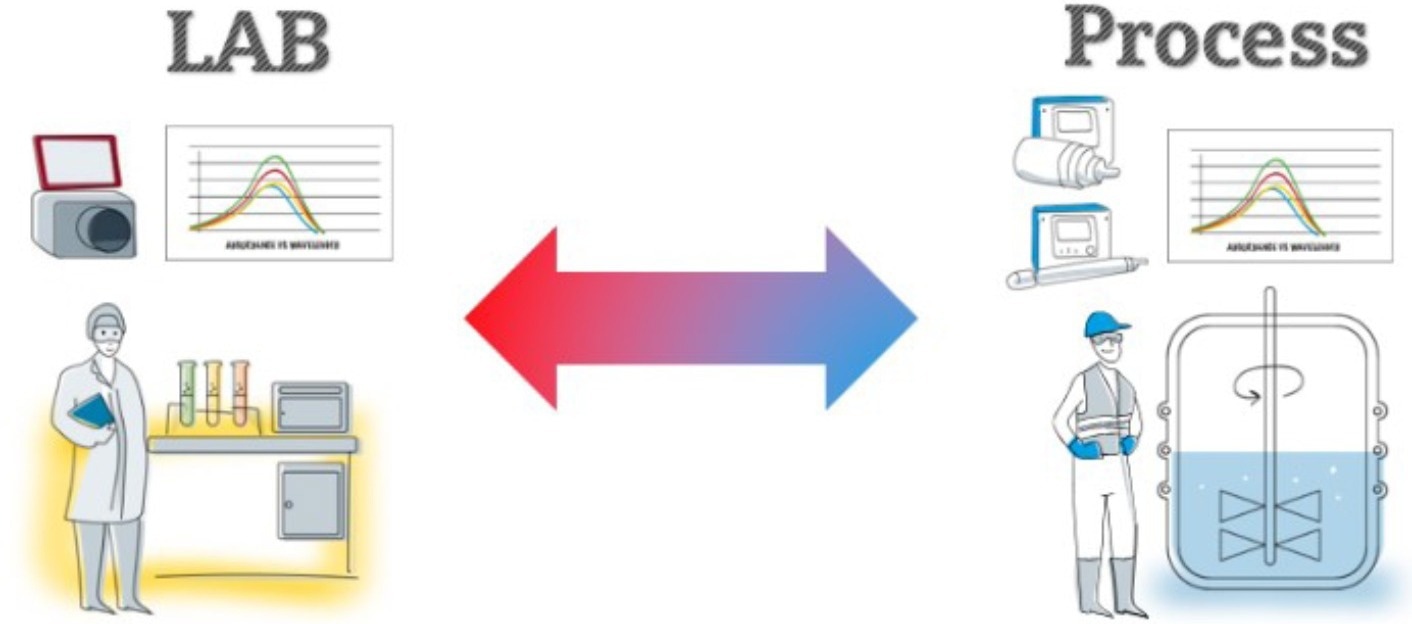
Figure 3. Process measurements must match lab test results. Image Credit: Endress+Hauser
Transfer of off-line measurements to the process
Off-line, at-line, and in-line measurements are frequently used when referring to the transfer of off-line data to the process.
Using a benchtop photometer to analyze photometric sample data is an illustration of off-line lab analysis. This offline method involves manually bringing a sample into the lab for benchtop instrument analysis.
It might or might not be necessary to prepare the sample before analysis. Due to the time required for sampling, delivering samples to the lab, and the timetable of activities taking place there, this kind of offline testing can take hours to complete.
With at-line (or on-line) analysis, a sample is taken directly from the process and put through an analysis using a field-tested analyzer, like a gas chromatograph. In this case, the analysis time is reduced from hours to minutes.
The time needed to bring the sample into the analyzer and any inherent analysis delay time is referred to as the analysis time. Field-hardened chromatographs are an example of an instrument of this type that nonetheless needs diligent maintenance to remain functional.
Utilizing an in-line sensor to measure a parameter right there in the process is known as in-line analysis. Usually, a transmitter receives a signal from a sensor that has been put into the process. The transmitter then creates a real-time measurement from the sensor signal and sends it to the automation system.
In-line Raman and tunable diode laser instruments, online sensors, and transmitters that use UV, visible, or near-infrared light technology are a few examples of this.
Use of consistent technology in the lab and in the process
Industrial processes now have greater opportunities when it comes to transitioning from the lab to the process and back with consistent sensor technology, thanks to the development of digital sensor technology for liquid analysis.
Digital communications are used between the sensor and the transmitter in digital sensor technology, including Memosens® from Endress+Hauser.
Figure 4. Digital sensor technology offers a number of benefits as compared to traditional analog approaches. Image Credit: Endress+Hauser

Figure 5. Lab sensors use the same digital technology as their process counterparts but can be packaged in plastic protective housings. Image Credit: Endress+Hauser
In addition to providing further performance advantages, this enhances measurement performance by reducing interference issues frequently present with analog sensors, like EMI/RFI interferences and ground loops. Additionally, digital sensor technology has internal sensor memory and sensor-to-cable links to make it easier to use (Figure 4).
Digital sensor technology, which was first used in process sensors, can now be used in laboratories. An identical sensor to the one used in the lab, for instance, can be used to take crucial pH values during the process.
As an alternative, a pH sensor with a safe plastic body can be used to take the same measurement in the lab, retaining uniformity and having a more durable design for frequent handling (Figure 5).
The same sensor technology that will be employed later in production can be used in the research lab as new medications and therapies are created. Additionally, the lab can employ the same equipment used in production to validate process measurement points by using smaller, more compact electrical devices to interact with the sensors (Figure 6).
Mobile handheld devices can be used for spot checks both in the lab and during the process, ensuring uniformity throughout.
These portable handheld devices frequently use Bluetooth technology and apps to communicate with digital sensors, and Bluetooth connections make it simple to transfer measured values to host automation systems.
Consistency is supplied from the lab to the process, thanks to sensors that are created for both the process and the lab, as well as electronics that can switch between the two.

Figure 6. Mobile handheld interface device for use with sensors in the lab or in the process. Image Credit: Endress+Hauser
Lab-based computer interface and sensor management
There are more complex systems accessible in addition to small transportable handheld devices for sensor interaction in the lab. For instance, it is possible to interface with a range of sensors for benchtop operation in the lab using PC-based sensor management software.
Generally, this kind of software will offer sample measurement, calibration of sensors, and management of the sensor life cycle (Figure 7).
Complete access to digital sensors in the lab is made possible by sensor management software. When a sensor is automatically detected, the software records any subsequent actions in the database together with the sensor’s serial number.
Even after the sensor has been detached from the system, sample measurements and sensor adjustments are kept for quick retrieval, and reports offer essential regulatory paperwork.
Data security, user management, and an extensive audit trail are all features of some kinds of sensor management software that can be licensed with 21 CFR Part 11 adherence.
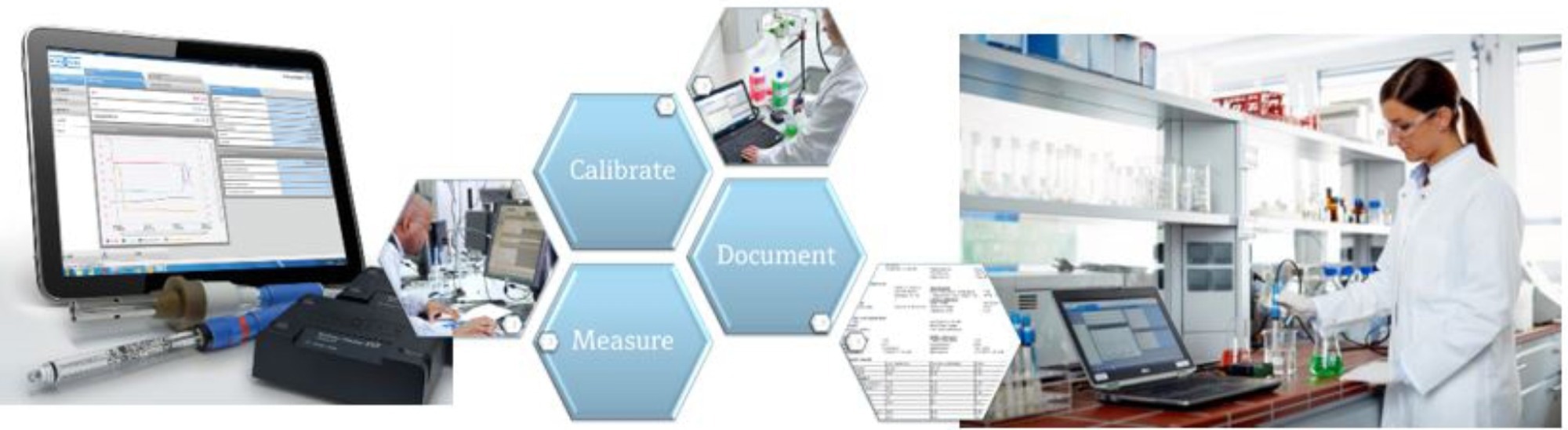
Figure 7. PC-based software is available to provide complete digital sensor management. Image Credit: Endress+Hauser
Conclusion
Labs are used to design products and inspect the quality of manufacturing procedures. Due to the compulsory role of quality assurance, a lab will be used to make many of the same measurements made in the production process.
It is becoming more and more crucial to adopt measuring technologies that are similar from lab-to-process to maintain consistency between process and lab results.
About Endress+Hauser

Endress+Hauser is a global leader in measurement instrumentation, services and solutions for industrial process engineering. We provide process solutions for flow, level, pressure, analytics, temperature, recording and digital communications, optimizing processes in terms of economic efficiency, safety & environmental impact.
As a trusted partner in Raman for over 30 years, Endress+Hauser has a long history in production, including GMP manufacturing, with many proven successes. Our unmatched expertisehigh-qualityty solutions, and exceptional customer service sets Kaiser far above any other Raman option in the marketplace. Endress+Hauser instrumentation, powered by Kaiser Raman technology is currently used throughout the chemical, food and beverage, oil and gas, pharmaceutical, and biopharmaceutical industries to optimize process efficiency and deliver quality products.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.