The baculovirus-insect cell expression system is a potent and adaptable platform for protein production. The robust post-translational modification capabilities and relatively high cell culture density of this platform make it a highly favorable choice for recombinant protein production. It is particularly well-suited for the expression of intracellular proteins, protein complexes, and viral proteins.
Sino Biological developed a unique culture medium called SIM SF, which is capable of supporting cell growth up to 6 × 106 cells/mL. It was specifically intended for insect cells. Additionally, Sino Biological can offer clients top-notch recombinant protein expression services using the baculovirus–insect cell system, thanks to improved expression vectors, virus packaging technology, and Sino Biological’s vast experience in recombinant protein expression and purification.
Flexible culture volumes
20 mL-1500 L
Volumes used for cell culture range from 20 mL to 1500 L.
High purity
> 95%
Completed projects
1000+
1,000+ proteins were successfully expressed and purified.
Workflow of baculovirus–insect cell expression
Sino Biological offers a large selection of widely used insect cell lines, including Sf9, Sf21, and Hi-5. For viral amplification, Sino Biological often utilizes Sf9 cells, and for protein expression, they use Hi-5 cells. Sino Biological has the ability to significantly boost protein expression levels and deliver highly active proteins through the utilization of optimized expression vectors and high-titer virus packaging technologies.
Technical workflow
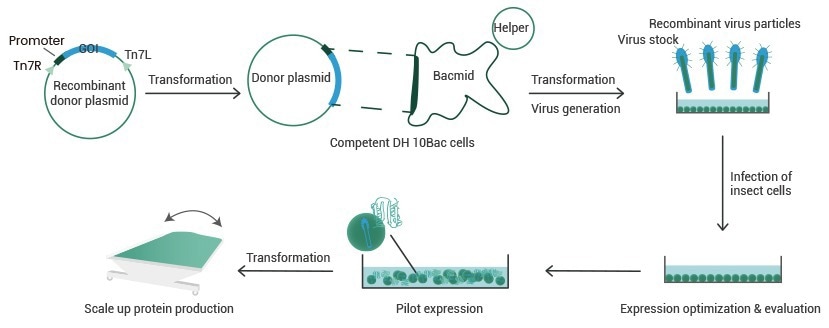
Flowchart of Baculovirus–Insect Cell Recombinant Expression. Image Credit: Sino Biological Inc.
Instruments

Bioreactors. Image Credit: Sino Biological Inc.

AKTA pure. Image Credit: Sino Biological Inc.

Odyssey. Image Credit: Sino Biological Inc.
Service details for insect cell expression
- Gene synthesis and codon optimization: 1-2 weeks
- Vector construction: 1-2 weeks
- Virus packaging and amplification: 2-3 weeks
- Protein expression and purification: 2-3 weeks
- Large-scale expression and purification (Optional): 3-5 weeks
Case studies of insect cell protein expression
Case#1: Removal of a highly hydrophobic region to enhance protein expression
In this scenario, the target protein's ECD (extracellular region) was expressed as a secreted protein. However, the construct including the entire ECD was not secreted. A hydrophobicity study of the target sequence revealed the presence of a significant non-transmembrane hydrophobic region at the protein’s N terminus.
Hydrophobicity, in conjunction with other structural properties (e.g., regions of high disorder, repeated amino acid motifs, etc.), could encourage instability in recombinant protein expression. These areas can be eliminated during construct optimization if they are not directly involved in protein function.
In this case, the target protein was effectively produced and purified to a purity of greater than 95 % after the hydrophobic region was removed from the protein sequence.
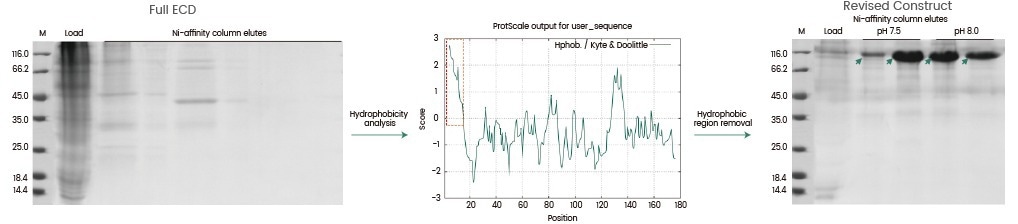
Image Credit: Sino Biological Inc.
Case#2: Purification procedure
In this case study, insect cells were used to express a single-pass transmembrane protein. Transmembrane proteins are influenced chemically by their surroundings. Variations in ionic strength, pH, oxidation state, and other characteristics can affect the stability of proteins. Additives might be needed during purification to stabilize the target protein or to promote tag exposure.
Detergent formula 1 produced a poor extraction of the target protein, as illustrated in the figure below, and no pure recombinant protein was produced. Protein extraction was subsequently improved by detergent formula adjustment. During the final polishing stage, detergent formula 2 was substituted with another detergent (DDM) to stabilize the final protein product.
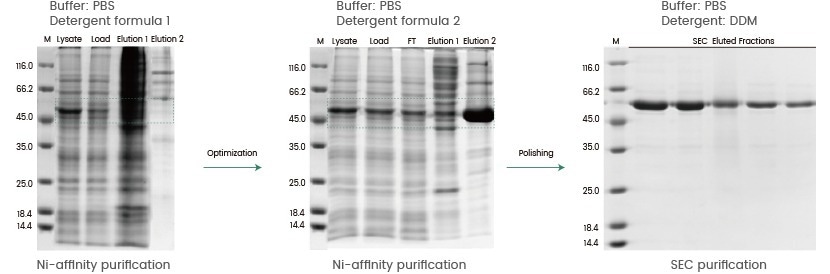
Image Credit: Sino Biological Inc.
Applications of baculovirus expression platform
Basic research

Image Credit: Sino Biological Inc.
Baculovirus is a crucial system for mega-complex expression since it is a vector that can carry a high number of exogenous genes. For fundamental research, including structural biology and protein functional studies, Sino Biological offers premium recombinant proteins through the high-performance baculovirus–insect cell expression technology.
IVD Reagent development

Image Credit: Sino Biological Inc.
With the correct protein post-translational modification, insect cells are able to express the majority of eukaryotic proteins. It is advantageous for producing bioactive raw materials because it can accommodate large exogenous genes. Sino Biological has created a platform for downstream validation in the process of developing raw materials for diagnostics.
Enzyme research
Image Credit: Sino Biological Inc.
Using this method, it is possible to obtain biologically active proteins since the proteins expressed in the baculovirus–insect cell platform fold properly. It is therefore possible to express active enzymes and mutants on the baculovirus–insect cell platform, which will aid in the research of enzymology and evolving enzymes.
Learn More About Expression System