Assessing the pharmacokinetics (PK) of antibody-drug conjugates (ADCs) in both preclinical and clinical settings is crucial for their development. Biotransformation processes can lead to the creation of different in vivo forms of ADCs, which may impact the drug-to-antibody ratio (DAR) or alter the payload and linker. Typically, PK assays focus on measuring conjugated antibodies, total antibodies, and the released payload.
Sino Biological has developed anti-payload antibodies that exhibit high purity, specificity, and affinity, which are instrumental in analyzing the plasma/serum PK profiles, DAR values, and drug load distribution in ADC drugs.
During the preclinical and clinical phases of ADC drug development, these payload antibodies are utilized to assess the safety and efficacy of ADCs, thereby expediting the development process.
Product features
- High purity
- > 95 % (SDS-PAGE and SEC-HPLC verified)
- High affinity
- Verified using the ADCs binding assay
- High specificity
- Precisely identifies payloads
Product list
Source: Sino Biological Inc.
| Target |
Cat# |
Product Name |
| MMAE |
68218-MM08 |
Anti-MMAE Antibody, Mouse MAb |
| MMAF |
68219-MM01 |
Anti-MMAF Antibody, Mouse MAb |
There are now four more anti-payload antibodies being developed: anti-DM1 antibody, anti-DM4 antibody, anti-SN-38 antibody, and anti-Dxd antibody.
Applications
ADC PK analysis
PK involves the study and characterization of a drug's four key phases as it travels through the human or animal body: absorption, distribution, metabolism, and excretion (ADME). Understanding and evaluating the PK profiles of antibody-drug conjugates (ADCs) is critical for their development, optimization, and effective clinical application.
ADC analyte testing methods
The ligand-binding assay (LBA) and liquid chromatography-mass spectrometry (LC-MS) are recommended methods for analyzing several ADC analytes. LBA usually detects total antibodies (DAR > 0) and conjugated antibodies (DAR ≥ 1), whereas LC-MS/MS detects drug molecules in different forms.
Source: Sino Biological Inc.
| Analyte type |
Analyte details |
Typical method |
| Conjugated antibody |
Antibody with minimum of DAR > 1 |
LBA |
| Total antibody |
Conjugated, partially unconjugated and
fully unconjugated (DAR ≥ 0) |
LBA |
| Antibody-conjugated drug |
Total small-molecule drug conjugated to antibody |
Affinity LC-MS/MS, LBA |
| Unconjugated drug |
Small-molecule drug not conjugated to antibody |
LC-MS/MS |
| Total drug |
Total unconjugated and conjugated drug |
LC-MS/MS |
Reference: DOI: 10.4155/bio.13.38
Current LBA formats for analyzing total and conjugated antibody levels
ELISA is a widely used LBA method for ADC PK assays. Anti-payload antibodies can be employed to detect the conjugated antibodies (DAR ≥ 1). The procedure is as follows: Immobilized anti-payload antibodies bind to the drug component of ADCs. Afterward, HRP-anti-human IgG Fc antibody, HRP-anti-ID antibody, or HRP-streptavidin-biotinylated target is added to detect the conjugated antibodies.
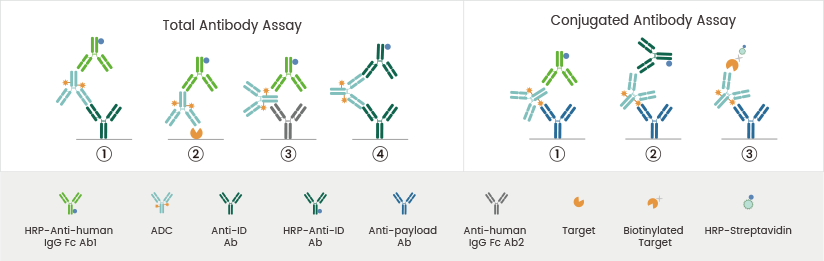
Reference: DOI: 10.3390/molecules27196299. Image Credit: Sino Biological Inc.
DAR value detection
ADCs' effectiveness is mostly governed by the concentration of drug molecules in tumor cells. The drug-to-antibody ratio (DAR) defines drug loading capacity and influences the PK characteristics of ADCs in vivo, such as distribution and clearance rate.
However, at high DAR levels, the drug clearance rate rises, potentially affecting the safety and effectiveness of ADCs. The precise determination and monitoring of the distribution of DAR values is crucial throughout the ADC development process.
The average DAR (the ratio of antibody-conjugated drug to total antibody) and the distribution of DAR (the percentage of ADCs with distinct DARs to total ADCs, respectively) are the two components of DAR analysis. Anti-payload antibodies in affinity LC-MS can be used to identify antibody-conjugated medications of ADCs.
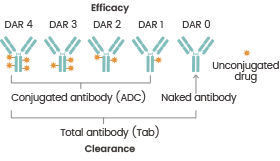
The relationship between DAR values, efficacy, and clearance. Reference: DOI: 10.1208/s12248-020-00475-8. Image Credit: Sino Biological Inc.
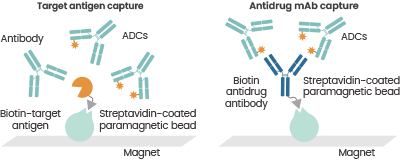
Characterization of DAR distributions utilizing affinity capture LC-MS. Reference: DOI: 10.4155/bio.12.299. Image Credit: Sino Biological Inc.
Product validation data
High purity
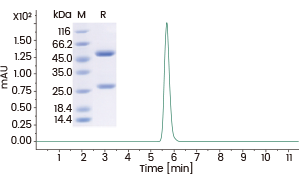
>95% as determined by SDS-PAGE and SEC-HPLC. Image Credit: Sino Biological Inc.
High affinity
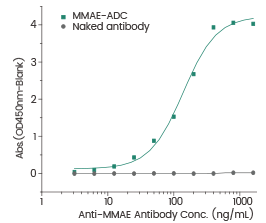
Immobilized MMAE-ADC at 1 μg/mL (100 μL/well) can bind the Anti-MMAE Antibody, the EC₅₀ of Anti-MMAE is 50 -1000 ng/mL. The naked antibody (without MMAE) is the negative control. Image Credit: Sino Biological Inc.
High specificity
Competitive assay
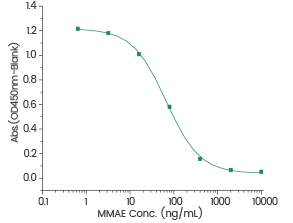
Immobilized Anti-MMAE Antibody at 2 μg/mL (100 μL/well), gradient-diluted free MMAE from 10 μg/mL (50 μL/well) block the binding of MMAE Antibody to MMAE-HRP 2 μg/mL (50 μL/well). (Validation Experiment). Image Credit: Sino Biological Inc.
Cross-reactivity validation
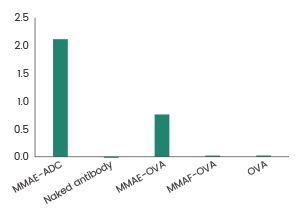
Immobilized MMAE-ADC, MMAE-OVA, MMAF-OVA, OVA at 1 μg/mL (100 μL/well), then add the Anti-MMAE Antibody to detect the cross-reactivity. (Validation Experiment). Image Credit: Sino Biological Inc.
Recommended products and services
Sino Biological provides an extensive toolset for detecting both total and conjugated antibodies in ELISA experiments. This includes anti-payload antibodies, ADC target proteins, and biotinylated proteins. To aid in ADC PK research, Sino Biological also provides anti-idiotype antibody creation services.
- Superior quality, including great sensitivity, affinity, and stability
- Excellent specificity and low cross-reactivity with human IgG
- A wide range of anti-idiotype antibodies enable accurate detection of total and unbound drug levels
- Various targeting modalities are available, including full-length mAb, F(ab')2, Fab, scFv, ADC, bsAb, Fc-fusion protein, and others
- High activity, purity, and consistent performance between batches
- Validation of biosimilar antibody binding
- The activity was validated using ELISA/SPR/BLI tests
- Multiple species are available for cross-reactivity testing
- Quality control follows ISO 9001/ISO 13485/GMP guidelines
- Site-specific biotinylated proteins
- Bioactivity was validated using ELISA, SPR, and BLI
- High lot-to-lot consistency
- Simple labeling with good detection sensitivity
- Multiple species are covered, including essential targets such as cell treatments, antibodies, Fc receptors, and ADCs
Learn More About Reagents Here!