As of September 27, 2022, the coronavirus disease 2019 (COVID-19) has been responsible for over 6.5 million deaths worldwide. Caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), COVID-19 has been remarkably unpredictable in its clinical severity.
As a result, many efforts have been made to determine the factors that increase an individual’s risk of severe or fatal COVID-19. A recent Pathogens journal study reviews the potential role of the gut microbiome in determining severe COVID-19.
Study: Gut Microbiota and COVID-19: Potential Implications for Disease Severity. Image Credit: nobeastsofierce / Shutterstock.com
Introduction
Previous research has emphasized the importance of the gut microbiome in regulating inflammatory processes at both local and systemic levels. Due to its widespread communication with other organs and systems within the body, the microbiome has also been referred to as the ‘second brain’ of the body.
The risk of gut dysbiosis has been shown to increase during SARS-CoV-2 infection, as well as post-acute COVID sequelae (PASC), the latter of which is more commonly known as ‘long COVID.’ In these situations, an increased concentration of opportunistic pathogenic species has been reported, while the number of ‘good’ bacteria declined. However, the reverse was also true, in which patients with fewer gut symptoms had lower chances of clinical deterioration.
Dysbiosis triggers inflammation
SARS-CoV-2 gains entry to the target host cell by binding its receptor binding domain (RBD) within the viral spike protein S1 subunit to the angiotensin-converting enzyme 2 (ACE2) receptor.
The ACE2 receptor also has a vital role in the renin-angiotensin-aldosterone system (RAAS), which regulates blood pressure. Therefore, the downregulation of ACE2 during SARS-CoV-2 infection can impact RAAS function.
The cytokine storm, characterized by the excessive release of cytokines, often occurs in severe COVID-19. This arises following the detection of infected cells by innate immune cells, which subsequently triggers a hyperinflammatory immune response. Cytokines release pro-inflammatory messenger molecules such as interleukin-1 (IL-1), IL-6, IL-8, IL-12, tumor necrotic factor α (TNF-α), and interferon γ (IFN-γ) at high levels into the circulation.
The cytokine storm then induces systemic vasodilation and increases vascular permeability, thus leading to thrombotic complications, pulmonary edema, and multi-organ failure. Critical and fatal outcomes are more likely when these occur in people with other risk factors for cardiovascular disease or thrombosis. The systemic injury and multi-organ dysfunction characterize acute respiratory distress syndrome (ARDS).
Dysbiosis triggers systemic effects
About one in five COVID-19 patients develop diarrhea, with or without abdominal discomfort or pain, at some point during the infection. Viral RNA appears to persist in the gut mucosa and stool of previously infected patients for over one month from symptom onset. In addition to causing direct injury to the gut cells, SARS-CoV-2 likely produces gut injury through this inflammatory environment.
Some COVID-19 patients with diarrhea exhibit higher serum cytokine levels, likely due to the viral protein binding to epithelial cell proteins that help form tight junctions. The resulting disruption of the gut epithelial barrier perturbs intracellular ion balance, thus causing colitis and inflamed gut walls. In addition, this may allow gut bacteria to cross over into the systemic circulation, thereby contributing to systemic immune-mediated inflammatory damage.
High ACE2 expression on the intestinal epithelium is key to ensuring a stable and healthy microbiome composition and function. Conversely, when ACE2 expression is reduced, sodium and amino acid uptake are also reduced, thus increasing susceptibility to intestinal inflammation.
Reduced ACE2 expression on intestinal epithelium weakens the mammalian target of the rapamycin (mTOR) pathway, thus altering anti-microbial peptides (AMPs) expression with increased autophagy. Interference with these interacting processes compromises enterocyte survival.
The weakened gut barrier results in chronic gut dysfunction and altered absorption of amino acids like phenylalanine, tryptophan, glutamine, and leucine. These effects can lead to inflammation, diarrhea, and dysbiosis.
Dysbiosis increases vulnerability to respiratory infection and its clinical severity. For example, mice with antibiotic-induced gut dysbiosis are more susceptible to influenza-induced lung inflammation.
Dysbiosis increases COVID-19 severity
Several previous studies have shown that COVID-19 is likely to be less severe in the presence of an increased abundance of seven bacterial classes, notably Faecalibacterium prausnitzii and Alistipes onderdonkii. Both of these microorganism species regulate tryptophan metabolism and immune homeostasis. Faecalibacterium prausnitzii levels are often low in Western diets but high in Mediterranean diets.
Severe COVID-19 has also been correlated with the presence of Coprobacillus, Clostridiumramosum, C.hathaway, and Erysipelotrichaceae. In addition, Coprobacillus is highly correlated with diarrhea and inflammation in irritable bowel disease (IBD).
Notably, several species of Bacteroides reduce ACE2 expression in the rat colon and are associated with less severe COVID-19.
Bacterial metabolites such as short-chain fatty acids (SCFAs) that originate from the bacterial fermentation of dietary fiber could also be relevant to homeostasis. Butyrate is particularly important as it maintains the integrity of the gut epithelial barrier while modulating immune and inflammatory pathways throughout the body.
SCFAs also act in many other ways to reduce inflammation. For example, gut dysbiosis reduces SCFA production by reducing the relevant bacterial taxa while promoting the abundance of opportunistic pathogens. These organisms can penetrate the weakened gut mucosal barrier, subsequently causing secondary infection in an already vulnerable individual and increasing their risk of severe or fatal outcomes.
COVID-19 triggers dysbiosis
In COVID-19 patients, the gut microbiome is altered, with fewer commensal bacteria of major species like Bacteroides, beneficial Lachnospiraceae, and Bifidobacterium. Simultaneously, opportunistic pathogens like Streptococcus, Rothia, and some species of Clostridia like C. hathawayi increase.
Increased signs of inflammation, such as C-reactive protein (CRP), lactate dehydrogenase (LDH), gamma-glutamyl transferase (GGT), and circulating IL-6, IL-10, IFN-γ, and TNF-α levels, have also been reported. These changes persisted even one month after viral clearance.
Probiotics reduce COVID-19 severity
Probiotics, especially those containing Bifidobacterium species, shorten upper respiratory infections through their anti-inflammatory effects that are mediated by their modulation of the gut microbiome. Thus, research indicates that probiotic supplementation could reduce COVID-19 severity.
Previously, probiotics increased virus-specific immunoglobulin M (IgM) and IgG without causing a significant change in the fecal microbiota profile. This indicates a direct action of IgG on immunity rather than through altered gut microbiome composition.
ACE and COVID-19 severity
Several studies have identified patients with pre-existing lung disease, cancer, solid transplants, older age (70 years or above), obesity, cardiovascular disease, and metabolic syndrome as having a higher risk of severe and fatal COVID-19. This could be due to changes in ACE2 activity involving RAAS. For example, lung cancer or diabetes patients express ACE2 at a higher baseline level.
SARS-CoV-2 infection causes increased ACE2 expression in the lungs and kidneys, especially in elderly patients and those with chronic lung disease, diabetes, and hypertension. This could lead to excessive activation of the ACE-Angiotensin II-angiotensin 1 receptor axis.
The consequences of these activities include vascular injury and dysfunction, inflammation and fibrosis of the lungs and heart muscle, kidney injury, and insulin resistance with increased oxidative stress.
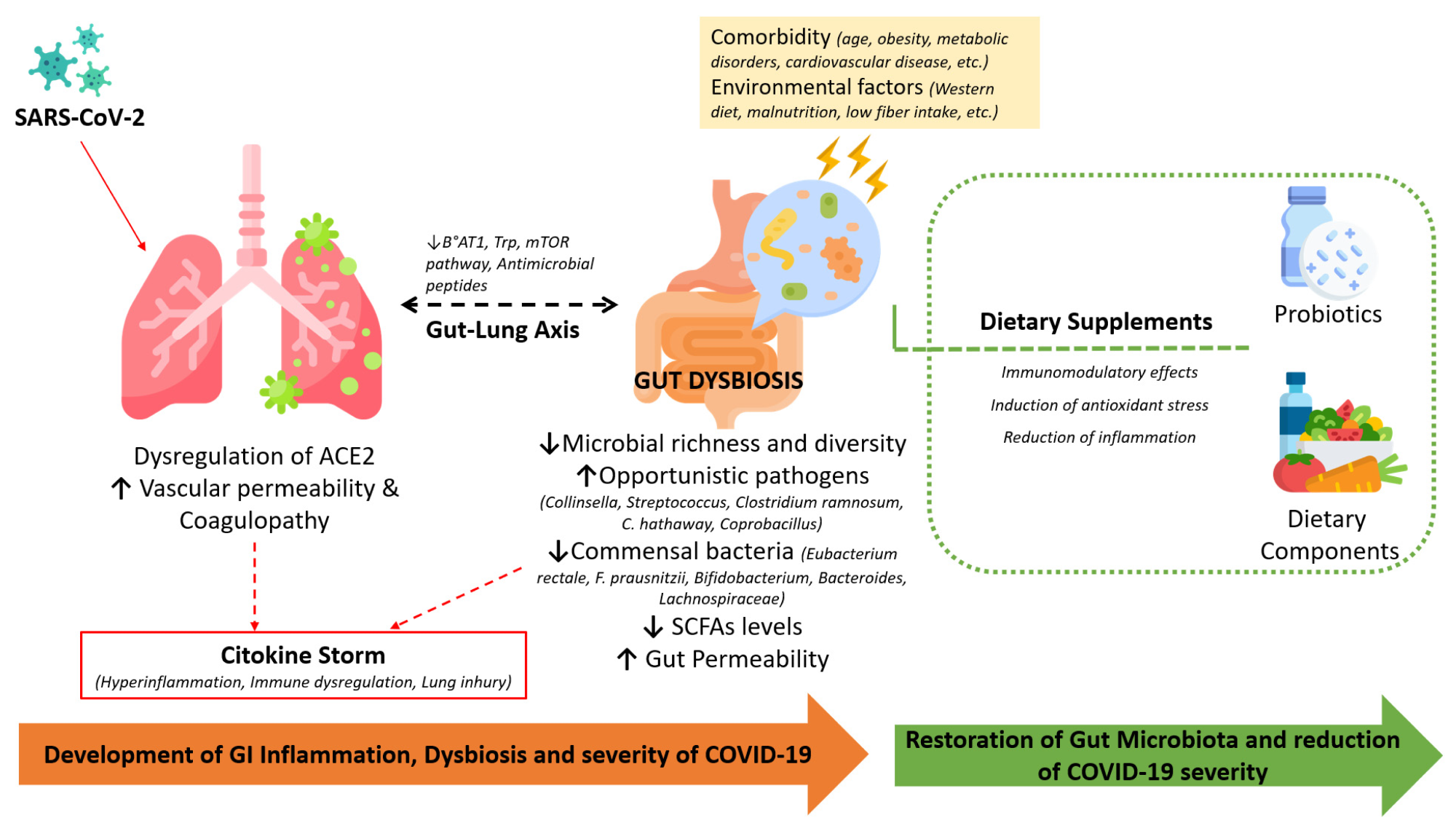 The involvement of the infection-induced dysregulation of ACE-2, comorbidities, and alterations in the gut microbiota during COVID-19 and the beneficial effects of dietary supplements in restoring the microbiota and immune homeostasis.
The involvement of the infection-induced dysregulation of ACE-2, comorbidities, and alterations in the gut microbiota during COVID-19 and the beneficial effects of dietary supplements in restoring the microbiota and immune homeostasis.
Nutrition and COVID-19
Obese individuals have chronic low-grade inflammation with high inflammatory mediators produced by visceral fat deposits and innate immune cells. This enhances the risk of cytokine storm after viral infection, further exacerbated by the accompanying gut dysbiosis and pressure on the lungs.
East Asian countries, many of which predominantly consume rice, reported much lower COVID-19 mortality rates than wheat-eating countries in the rest of the world.
Vitamin D modulates T-cell function, subsequently reducing inflammatory mediators while increasing anti-inflammatory molecules like IL-10 and its action on innate immune function. Supplementation with vitamin D could reduce the risk of severe infection and death related to COVID-19.
Vitamins C, E, and A, as well as metals like zinc and iron, are potent anti-inflammatory and antioxidant compounds that act through various pathways. Maintaining omega-3 fatty acids at an adequate level while preserving their ratio with omega-6 fatty acids at 1:5, respectively, is a potentially crucial protective measure. Overall, a plant-based diet enriched with functional foods and supplements could help protect against respiratory infections.
Conclusions
The gut microbiome composition significantly determines how the immune system responds to multiple pathogens, including SARS-CoV-2. Furthermore, dysbiosis can be considered a risk factor for and the result of severe COVID-19.
It is clear that interventions aiming to re-establish a correct microbiota composition are important for developing a more holistic approach to managing a series of diseases, including COVID-19….strategies aimed at lifestyle and dietary modifications can help to positively modulate the gut microbiome and play a preventive role in SARS-CoV-2 viral infection.”
Journal reference:
- Rocchi, G., Giovanetti, M., Benedetti, F., et al. (2022). Gut Microbiota and COVID-19: Potential Implications for Disease Severity. Pathogens. doi:10.3390/pathogens11091050.