Cancer is an international health problem. Approximately 19.3 million new cancer cases and almost 10 million cancer deaths occurred in 2020 globally, according to GLOBOCAN 2020 estimates of cancer incidence and mortality as noted in the International Agency for Research on Cancer report (Figure 1).1
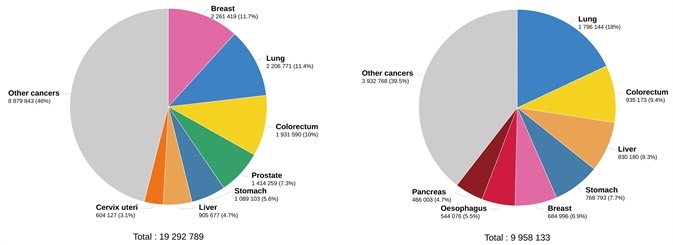
Figure 1. Estimated number of new cases in 2020. worldwide, both sexes, all ages (left), and estimated number of deaths in 2020. worldwide, both sexes, all ages (right). (Data source: GLOBCAN 2020, Graph production: Global Cancer Observatory (http://gco.iarc.fr/)). Image Credit: Sino Biological Inc.
Today, there are a number of treatments that patients can access, including chemotherapy, targeted therapy and immunotherapy. Targeted therapy is a category of precision medicine in which proteins that influence the division, growth and spread of cancer cells are primarily targeted.2
The BRAF V600E mutation, BCR-ABL fusion protein, HER-2 and epidermal growth factor receptor (EGFR) are among the key targets of targeted therapies.3 The majority of targeted drugs can be more or less split into two categories: small molecules and monoclonal antibodies.4
For instance, the US Food and Drug Administration (FDA) has approved trastuzumab (Herceptin®), a monoclonal antibody used to treat particular breast and gastric cancers that overexpress HER-2.5
Tyrosine kinase inhibitor gefitinib (Iressa®) is a small molecule, also approved by the FDA, that is used in the early treatment of metastatic non-small cell lung cancer. The target of this drug is EGFR exon 19 deletions or exon 21 (L858R) substitution mutations, as identified by an FDA-approved test.6
Immunotherapy has the capacity to harness the immune system’s own ability to fight cancer. In several solid tumors, immunotherapy is used as the first line of treatment.7
The checkpoint proteins of the immune system can be deemed as powerful therapeutic targets.8 For instance, on the T cell surface, the programmed cell death protein-1 (PD-1) is a checkpoint protein, and the programmed death-ligand 1 (PD-L1) is an immune checkpoint protein overexpressed on the tumor cell surface.
How PD-1 and PD-L1 interact enables tumor cells to dodge T cell-mediated immune responses. A number of antibody-based PD-1/PD-L1 blockade therapies have exhibited several benefits in the treatment of many human cancers.9
The FDA has previously approved therapeutic antibodies targeting immune checkpoint components (Table 1).10
Table 1. Checkpoint targets and their FDA-approved therapeutics. Source: Sino Biological Inc.
| Target |
FDA-approved therapeutic |
| PD-1 |
• nivolumab (Opdivo®)
• pembrolizumab (Keytruda®) |
| PD-L1 |
• atezolizumab (Tecentriq®)
• durvalumab (Imfinzi®)
• avelumab (Bavencio®) |
Presently, researchers are evaluating new targets, including T-cell immunoreceptor with Ig and ITIM domains (TIGIT). In mouse models of cancer, antibody co-blockade of TIGIT and PD-L1 amplifies CD8+ T cell effector function and represses tumor growth.11
In January 2021, predicated on data from the Phase II CITYSCAPE trial, Roche declared that the combination of tiragolumab (an anti-TIGIT antibody) and atezolizumab demonstrated promising efficacy and safety results in PD-L1-positive metastatic non-small cell lung carcinoma.12
Both small-molecule targeted therapies and immunotherapies have demonstrated very respectable clinical results. Furthermore, combining these two approaches offers the potential to stimulate synergistic and durable outcomes in patients.
For instance, results from a randomized phase II clinical trial of combined dabrafenib (targeting BRAF V600E), trametinib (targeting BRAF V600E or V600K) and pembrolizumab displayed promising results, including better progression-free survival and improved response, in patients who were given all three drugs compared to those who only received dabrafenib and trametinib.
However, the triple combination also revealed a series of adverse effects.13
Recombinant drug target proteins
As the data in Figure 2 shows, lung cancer is the primary cause of cancer deaths amongst both sexes, with around 1.8 million deaths (18%), followed by colorectal (9.4%), liver (8.3%) and stomach (7.7%) cancers. The most commonly seen type of lung cancer is non-small cell lung cancer (NSCLC).
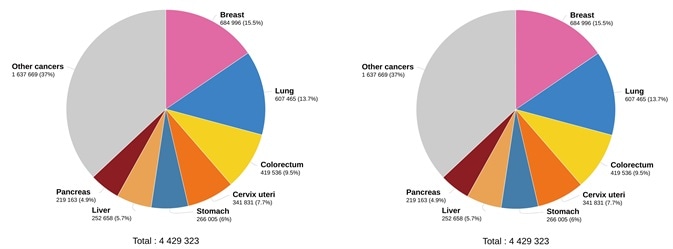
Figure 2. Estimated number of deaths in 2020. worldwide, both sexes, all ages (left), and estimated number of deaths in 2020. worldwide, females, all ages (right). (Data source: GLOBCAN 2020, Graph production: Global Cancer Observatory (http://gco.iarc.fr/)). Image Credit: Sino Biological Inc.
Across the world, breast cancer is the most deadly cancer in women (15.5%) (Figure 2). EGFR has been involved in the pathogenesis of numerous human malignancies, including colorectal cancer (CRC), NSCLC, head and neck squamous cell carcinoma (HNSCC) and breast cancer.14
This data has motivated the development of several FDA-approved EGFR inhibitors against such cancers.15 The extracellular domain of EGFR is a target of the EGFR ligand-blocking antibodies cetuximab and panitumumab.14
Gefitinib and erlotinib are tyrosine kinase inhibitors that interact with the intracellular domain of EGFR.16 Therefore, it is recommended to develop products that are comprised of the extracellular or intracellular domain of these proteins (Figure 3).
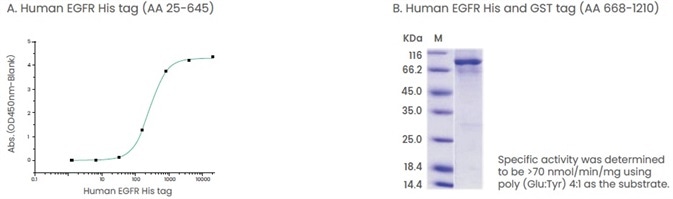
Figure 3. Examples of products that contain extracellular domain or intracellular domain of proteins. (A) The binding assay of EGF and EGFR. Immobilized human EGF hFc (Cat: 10605-H01H) at 2 μg/ml (100 μl/well) can bind human EGFR His (Cat: 10001-H08H). (B) The specific activity assay of EGFR (AA 668-1210). Image Credit: Sino Biological Inc.
Tumor cells are able to bypass detection by protecting themselves against the immune system. This is made possible by the activation of signaling pathways via ligand-receptor binding. The receptor-ligand interactions weaken antitumor immunity.17
For instance, in several types of cancers, the binding of the CD47 (ligand) to signal regulatory protein α (SIRPα) (receptor) activates an inhibitory signaling pathway that allows malignant cells to evade phagocytic elimination by macrophages.18
Several immune checkpoint therapeutic antibodies enhance antitumor activity by impeding ligand-receptor binding. Therapeutic antibodies blocking PD-1 and its ligand PD-L1 have previously shown clinical efficacy,19 and anti-CD47 antibodies have demonstrated the ability to boost the phagocytosis of tumor cells by impeding the binding of CD47 to SIRPα.18
Therefore, when designing tools to support these research efforts, it is absolutely critical to take ligand-receptor pairings into consideration (Figure 4).
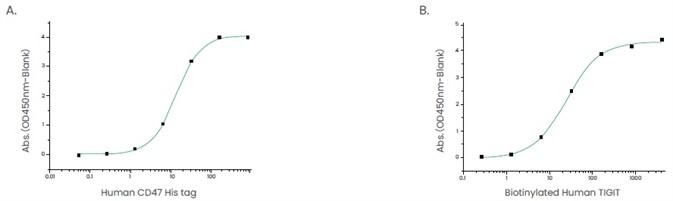
Figure 4. Examples of ligand-receptor pair proteins. (A) The binding assay of CD47 and SIRP_. Immobilized human SIRP hFc (Cat: 11612-H02H1) at 2 μg/ml (100 μl/well) can bind human CD47 His (Cat: 12283-H08H). (B) The binding assay of TIGIT and CD155. Immobilized human CD155/PVR His (Cat: 10109-H08H) at 2 μg/mL (100 μl/well) can bind biotinylated human TIGIT (hFc & AVI Tag) (Cat: 10917-H41H-B). Image Credit: Sino Biological Inc.
Cytokines
It may be the case that functional assays of antibodies must be tested on cells. Cytokines are generally used in such functional assays. For instance, TIGIT is expressed on T and NK cells, which are classed as antitumor effectors.20,21
TIGIT impedes the killing effect of NK cells on tumor cells, whereas the function of NK and T cells is restored by blocking TIGIT.22
When conducting NK cell and TIGIT blockade functional assays, cytokine IL-12 or a cocktail of cytokines (IL-12, IL-15, and IL-18) are added to stimulate NK cells. 22,23 An array of cytokines has been developed to facilitate a series of functional assays.
References
- Hyuna Sung et al., “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians n/a, no. n/a, accessed February 7, 2021, https://doi.org/10.3322/caac.21660.
- “Targeted Therapy for Cancer - National Cancer Institute,” cgvArticle, August 15, 2014, nciglobal,ncienterprise, https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies.
- William N. Hait, “Targeted Cancer Therapeutics,” Cancer Research 69, no. 4 (February 15, 2009): 1263–67, https://doi.org/10.1158/0008-5472.CAN-08-3836.
- Lei Zhong et al., “Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives,” Signal Transduction and Targeted Therapy 6, no. 1 (2021): 1–48, https://doi.org/10.1038/s41392-021-00572-w.
- “Herceptin (Trastuzumab) FDA Approval History,” Drugs.com, accessed January 10, 2022, https://www.drugs.com/history/herceptin.html.
- “Iressa (Gefitinib) FDA Approval History - Drugs.Com,” accessed January 10, 2022, https://www.drugs.com/history/iressa.html.
- Raju K. Vaddepally et al., “Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence,” Cancers 12, no. 3 (March 20, 2020), https://doi.org/10.3390/cancers12030738.
- P. Zatloukalová et al., “[The Role of PD-1/PD-L1 Signaling Pathway in Antitumor Immune Response],” Klinicka Onkologie: Casopis Ceske a Slovenske Onkologicke Spolecnosti 29 Suppl 4, no. Suppl 4 (Fall 2016): 72–77.
- Hyun Tae Lee et al., “Molecular Mechanism of PD-1/PD-L1 Blockade via Anti-PD-L1 Antibodies Atezolizumab and Durvalumab,” Scientific Reports 7 (July 17, 2017), https://doi.org/10.1038/s41598-017-06002-8.
- Hyun Tae Lee, Sang Hyung Lee, and Yong-Seok Heo, “Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology,” Molecules 24, no. 6 (March 26, 2019), https://doi.org/10.3390/molecules24061190.
- Francesco Passiglia et al., “Immune-Checkpoint Inhibitors Combinations in Metastatic NSCLC: New Options on the Horizon?,” ImmunoTargets and Therapy 10 (February 5, 2021): 9–26, https://doi.org/10.2147/ITT.S253581.
- “Genentech’s Novel Anti-TIGIT Tiragolumab Granted FDA Breakthrough Therapy Designation in Combination With Tecentriq for PD-L1-High Non-Small Cell Lung Cancer,” January 5, 2021, https://www.businesswire.com/news/home/20210104005887/en/Genentech%E2%80%99s-Novel-Anti-TIGIT-Tiragolumab-GrantedFDA-Breakthrough-Therapy-Designation-in-Combination-With-Tecentriq-for-PD-L1-High-Non-Small-Cell-Lung-Cancer.
- Johann S. Bergholz et al., “Integrating Immunotherapy and Targeted Therapy in Cancer Treatment: Mechanistic Insights and Clinical Implications,” Clinical Cancer Research 26, no. 21 (November 1, 2020): 5557–66, https://doi.org/10.1158/1078-0432.CCR-19-2300.
- Deric L. Wheeler, Emily F. Dunn, and Paul M. Harari, “Understanding Resistance to EGFR Inhibitors—Impact on Future Treatment Strategies,” Nature Reviews. Clinical Oncology 7, no. 9 (September 2010): 493–507, https://doi.org/10.1038/nrclinonc.2010.97.
- Remah Ali and Michael K. Wendt, “The Paradoxical Functions of EGFR during Breast Cancer Progression,” Signal Transduction and Targeted Therapy 2, no. 1 (2017): 1–7, https://doi.org/10.1038/sigtrans.2016.42.
- “Contribution of EGFR and ErbB-3 Heterodimerization to the EGFR Mutation-Induced Gefitinib- and Erlotinib-Resistance in Non-Small-Cell Lung Carcinoma Treatments,” PLOS ONE 10, no. 5 (May 20, 2015): e0128360, https://doi.org/10.1371/journal.pone.0128360.
- H. Harjunpää and C. Guillerey, “TIGIT as an Emerging Immune Checkpoint,” Clinical & Experimental Immunology 200, no. 2 (2020): 108–19, https://doi.org/10.1111/cei.13407.
- Wenting Zhang et al., “Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis,” Frontiers in Immunology 11 (2020), https://doi.org/10.3389/fimmu.2020.00018.
- Zatloukalová et al., “[The Role of PD-1/PD-L1 Signaling Pathway in Antitumor Immune Response].”
- Theresa L. Whiteside and Ronald B. Heberman, “Antitumor Effector Cells in Humans,” Holland-Frei Cancer Medicine. 6th Edition, 2003, https://www.ncbi.nlm.nih.gov/books/NBK13070/.
- “TIGIT - an Overview | ScienceDirect Topics,” accessed March 29, 2021, https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/tigit.
- Fanqiao Meng et al., “Overexpression of TIGIT in NK and T Cells Contributes to Tumor Immune Escape in Myelodysplastic Syndromes,” Frontiers in Oncology 10 (2020), https://doi.org/10.3389/fonc.2020.01595.
- Xiaowan Yin et al., “Expression of the Inhibitory Receptor TIGIT Is Up-Regulated Specifically on NK Cells With CD226 Activating Receptor From HIV-Infected Individuals,” Frontiers in Immunology 9 (2018), https://doi.org/10.3389/fimmu.2018.02341.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.