DPX extraction, a dispersive SPE method, requires extremely less solvent and does not involve complicated and time-consuming steps when compared to other SPE methods. DPX extraction is an established and highly reproducible method and offers the required sensitivity for clinical and forensic purposes.
DPX mixed-mode tips provide a simple, accurate, and rapid method to extract urine samples and allow the analysis of drugs of abuse in these samples. The Integra Viaflo 96 system enables high-throughput, semi-automated sample processing. A single 96-well plate with loaded samples can be extracted and prepared for LC-MS/MS injection in less than 10 minutes, without any need for evaporation.
Equipment
Analysis was carried out on a Thermo TSQ Vantage triple quadrupole device with an Agilent 1260 HPLC, using an Agilent Poroshell EC-C18 column (3.0 x 50 mm, 2.7 µm) with a 10 µL injection. Then, extractions were carried out on an Integra Viaflo 96 (part# 6001) with a 300 µL head (part# 6103) and with the 3-position stage (part # 6230) using DPX mixed-mode tips (2 mg WAX, 1 mg RP).

Experiment
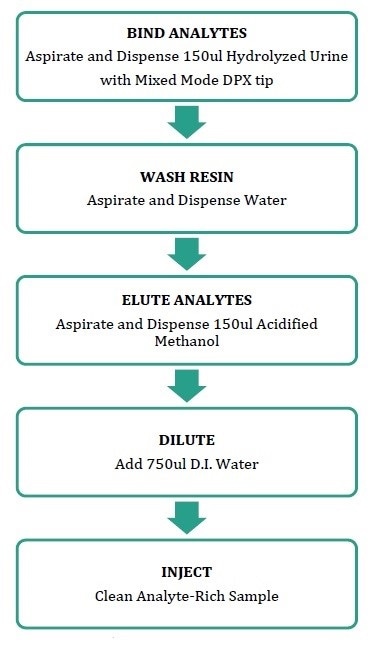
The Integra system is loaded with well plates containing hydrolyzed urine samples. Then, pre-filled well pates for elution solvent and water wash are mounted into the Integra, and 30% methanol is aspirated from a solvent reservoir to condition the DPX tips.
After conditioning, the DPX tips aspirates and dispenses the sample solutions (150 µL urine, 250 µL total volume (enzyme, buffer, internal standard mix)) three times so as to bind the drugs of abuse to the sorbent. Then, water is aspirated and dispensed, and the target analytes are eluted by aspirating and dispensing the 1% formic acid in methanol for three times.
Results and discussion
From beginning to end, the semi-automated dispersive SPE method takes less than 10 minutes to prepare a single 96-well plate. This method provides reproducible, accurate, and linear results. It was observed that all correlation coefficients were more than 0.99 for the range of 12.5-400 ng/mL, with the majority of analytes being linear from 6.25 to 800 ng/mL.
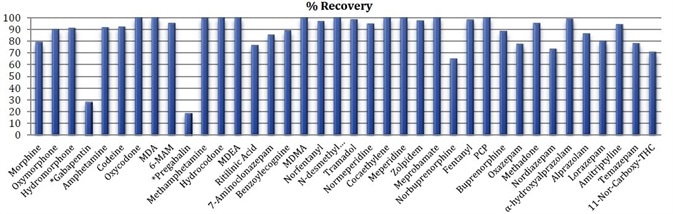
Using 4 replicate extractions at 400 ng/mL, relative standard deviations were measured and ranged from 1.1 to 9.3%. Limits of detection (LOD) were determined as 3.3*σ/m, where m indicates the slope of the calibration line and σ represents the standard deviation of the lowest non-zero calibrator. Limits of quantitation (LOQs) were determined as 10*σ/m. LOD and LOQ ranged from 0.50 to 13 ng/mL and 1.5 to 38 ng/mL, respectively.
It must be noted that the LOQ and LOD largely depend on the sensitivity of the LC/MS method and instrumentation. If sensitivity has to be improved to reduce LOQs and LODs, the extracts can be concentrated using solvent evaporation. Additionally, the elution volume can be raised to 500 µL in order to improve recoveries, or a larger volume of sample solution can be extracted to achieve lower LODs and LOQs.
|
Compound
|
R2
|
% RSD (n=4)
|
LOD (ng/mL)
|
LOQ (ng/mL)
|
|
Morphine
|
0.9974
|
5.6
|
1.5
|
4.5
|
|
Oxymorphone
|
0.9982
|
4.9
|
2.5
|
7.5
|
|
Hydromorphone
|
0.9982
|
3.0
|
5.7
|
17.1
|
|
Gabapentin
|
0.9989
|
1.7
|
10
|
30
|
|
Amphetamine
|
0.9944
|
3.1
|
9.3
|
28.2
|
|
Codeine
|
0.9972
|
7.1
|
7.7
|
23.1
|
|
Oxycodone
|
0.9968
|
8.0
|
9.8
|
28.9
|
|
MDA
|
0.9940
|
6.4
|
13
|
39
|
|
6-MAM
|
0.9932
|
4.6
|
1
|
3
|
|
Pregabalin
|
0.9972
|
1.1
|
10
|
30
|
|
Methamphetamine
|
0.9993
|
5.3
|
12.7
|
38.1
|
|
Hydrocodone
|
0.9949
|
2.2
|
6.5
|
19.5
|
|
Nortriptyline
|
0.9943
|
6.7
|
10
|
30
|
|
Ritilinic Acid
|
0.9945
|
5.2
|
3.5
|
10.3
|
|
7-Aminoclonazepam
|
0.9959
|
1.3
|
3.8
|
11.2
|
|
Benzoylecognine
|
0.9943
|
2.4
|
5.5
|
16.4
|
|
MDMA
|
0.9975
|
1.7
|
11.8
|
35.3
|
|
Norfentanyl
|
0.9970
|
7.9
|
2
|
6
|
|
tramadol
|
0.9956
|
9.3
|
9
|
28
|
|
Tramadol
|
0.9962
|
4.5
|
8.7
|
26
|
|
Normeperidine
|
0.9937
|
3.3
|
5
|
15.7
|
|
Cocaethylene
|
0.9981
|
8.9
|
8.2
|
24.8
|
|
Meperidine
|
0.9960
|
3.3
|
4.4
|
13.2
|
|
Zolpidem
|
0.9975
|
3.4
|
7.8
|
23.4
|
|
Cyclobenzaprine
|
0.9973
|
3.4
|
10
|
30
|
|
Norbuprenorphine
|
0.9912
|
4.9
|
3
|
10
|
|
Fentanyl
|
0.9979
|
3.4
|
.5
|
1.5
|
|
PCP
|
0.9967
|
4.7
|
1
|
4
|
|
Buprenorphine
|
0.9914
|
7.6
|
1
|
4
|
|
Oxazepam
|
0.9981
|
7.7
|
4.4
|
13.1
|
|
Methadone
|
0.9932
|
3.1
|
7.7
|
23.2
|
|
Nordiazepam
|
0.9970
|
7.4
|
7.2
|
21.7
|
|
α-hydroxyalprazolam
|
0.9922
|
6.3
|
6.4
|
19.2
|
|
Alprazolam
|
0.9937
|
3.7
|
13
|
40
|
|
Lorazepam
|
0.9984
|
3.7
|
2.4
|
7.3
|
|
Amitriptyline
|
0.9983
|
3.6
|
10
|
30
|
|
Temazepam
|
0.9954
|
4.3
|
6.8
|
20.3
|
|
11-Nor-Carboxy-THC
|
0.9985
|
9.3
|
4
|
12
|
Conclusion
The dispersive SPE method described in this article provides the required sensitivity, recoveries, and sensitivity for a consistent sample preparation method in a high-throughput environment. The analysis is made fast and non-tedious, thanks to semi-automation of the sample preparation process.
About INTEGRA
 INTEGRA provides innovative solutions for Liquid Handling and Media Preparation applications which serve the needs of their customers in research, diagnostics and quality control laboratories.
INTEGRA provides innovative solutions for Liquid Handling and Media Preparation applications which serve the needs of their customers in research, diagnostics and quality control laboratories.
Their instruments and plastic consumables are developed and manufactured in Zizers, Switzerland and Hudson, NH USA. In order to remain close to their customers, they maintain a direct sales and support organization in North America, the UK, France and Germany, as well as a network of over 100 highly trained distribution partners worldwide.
In recent years they have focused on developing a new and technologically advanced range of handheld electronic pipettes which are simple to use and meet the ergonomic needs of their customers.
Today they are proud to offer the widest range of electronic pipettes in the market spanning a range from single channel pipettes up to 384 channel bench-top instruments.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and iform site visitors interested in medical research, science, medical devices and treatments.