Sponsored Content by AbcamDec 15 2017
In the mammalian CNS, the principle excitatory neurotransmitter is L-Glutamate, which acts through G-protein coupled (metabotropic) receptors and ligand gated ion channels (ionotropic receptors). These receptors play a key role in excitatory synaptic plasticity and synaptic transmission, which are responsible for memory and learning.
Ionotropic Glutamate Receptors (iGluRs)
iGluRs are ligand-gated ion channels, mediating the majority of excitatory neurotransmission in the brain.
Structure of iGluRs
iGluRs are present in pre- and postsynaptic cell membranes, mainly in the CNS1. They are classified into kainate receptors, NMDA receptors and AMPA receptors. The naming of these subfamilies is based on their affinities for the synthetic agonists, kainic acid (kainate), NMDA (N-methyl-d-aspartate) and AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate)2. The delta receptor family has been categorized as an iGluR by sequence homology3 (Figure 1).
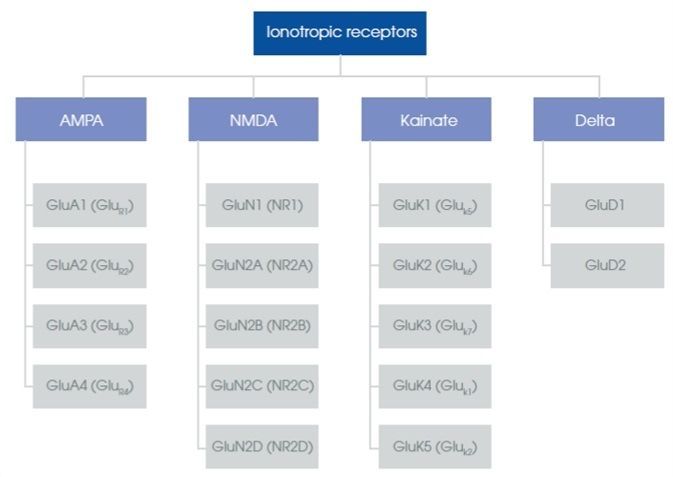
Figure 1. Diagram of the ionotropic glutamate receptor subgroups.
Similar to other ligand-gated ion channels, four domains are present in iGluRs:
- The extracellular amino-terminal domain (ATD)
- The extracellular ligand-binding domain (LBD)
- Four transmembrane domains (TMD)
- An intracellular carboxyl-terminal domain (CTD)
The second TMD (TMII) consists of a re-entrant loop that gives rise an intracellular C-terminus and an extracellular N-terminus (Figure 2).
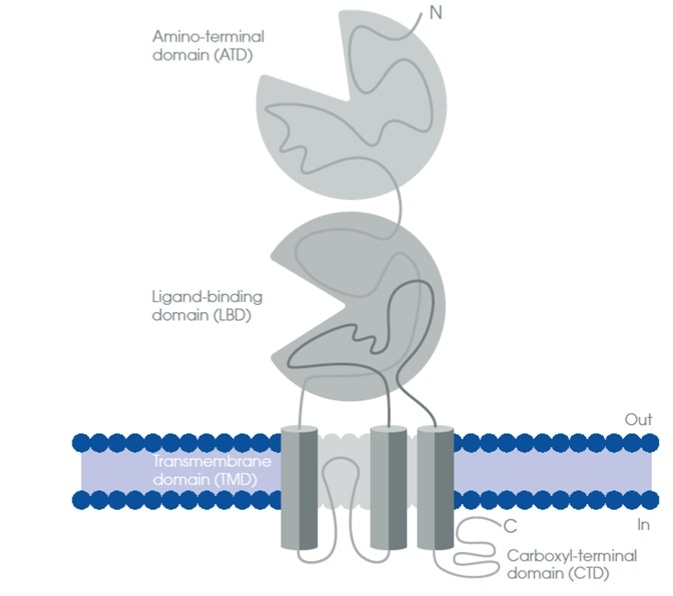
Figure 2. Schematic structure of the ionotropic glutamate receptors. (Adapted from Traynelis, S. F. et al., 2010)
Function
Fast excitatory neurotransmission is mediated by iGluRs. These receptors are responsible for synaptic plasticity and the capacity of humans to learn and form memories. iGluRs are nonselective cation channels, allowing ions like Ca2+, K+, or Na+ to pass through the channel when bound with glutamate1,4. An action potential (AP) is generated when a significant number of iGluRs are activated.
After the receipt of this signal, glutamate is removed from the synaptic cleft by excitatory amino acid transporters (EAATs) and as a result, the signal is effectively turned off in preparation for subsequent APs.
Excitotoxicity can occur when iGluRs are stimulated for a prolonged time as over-stimulation results in an abnormal membrane voltage potential. Glutamate uptake of EAATs is inhibited by this potential. Excitotoxicity plays a key role in nervous system injuries and neurodegenerative disorders and as a result, iGluRs become an interesting target for a number of therapeutic developments5.
References and Further Reading
- Purves, D. et al. in Neuroscience (eds. Purves, D. et al.) (Sinauer Associates, 2001)
- Alexander, S. P. H. et al. The Concise Guide to Pharmacology 2013/14: Ligand-gated ion channels. Br. J. Pharmacol. 170, 1706–1796 (2013).
- Orth, A., Tapken, D. & Hollmann, M. The delta subfamily of glutamate receptors: Characterization of receptor chimeras and mutants. Eur. J. Neurosci. 37, 1620–1630 (2013).
- Traynelis, S. F. et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496 (2010).
- Willard, S. S. & Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 9, 948–959 (2013).

About Abcam
Abcam is a global life sciences company providing highly validated antibodies and other binders and assays to the research and clinical communities to help advance the understanding of biology and causes of disease.
Abcam’s mission is to serve life scientists to help them achieve their mission faster by listening to their needs, continuously innovating and improving and by giving them the tools, data and experience they want. Abcam’s ambition is to become the most influential life science company for researchers worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.