Sponsored Content by AbcamJun 6 2019
RabMAb Products that Use Anti-polyethylene Glycol (PEG)
A process called PEGylation (covalent binding with polyethylene glycol, PEG) is frequently adopted to alter some characteristics of many proteins and drugs2-4. PEG is a synthetic polymer composed of repeating ethylene glycol (-CH2-CH2-O-) units. Its desirable properties include lack of toxicity, absence of charge, biocompatibility, intense hydrophilicity, and the large exclusion volume it shows when added to an aqueous solution.
Advantages of Using PEGylation
Some of the advantages of adding PEG include:
- Better bioavailability
- Improved circulation of the drug in blood
- Optimal pharmacokinetics
- Lower immune reactions
- Fewer administrations
Since PEG carries no charge and is biocompatible, it is not antigenic, and so antibodies cannot be raised against it. Abcam has developed a process after much research, which allows the creation of high-quality PEG RabMAb® antibodies of a large variety.
Follow these links to know more about each of these topics:
Anti-PEG Rabbit Monoclonal Antibodies
Once a biopharmaceutical is PEGylated, its hydrodynamic radius goes up, which means that it becomes less immunogenic and less susceptible to cleavage by proteolytic enzymes. In addition, it is excreted at lower rates through the kidneys, is more stable to proteolysis, and more soluble in aqueous solution.
It is possible to use an anti-PEG antibody in order to assess the pharmacokinetics of a drug over time, including its distribution, metabolism and excretion. It is also useful when techniques like ELISA, WB and IHC using PEGylated molecules have to be run through quality control.
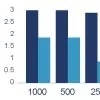
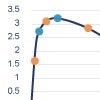

Case study: Rabbit versus mouse PEG antibody comparison
Case study: Pharmacokinetics usage with PEG RabMAb products
Case study: Immunohistochemistry usage with PEG RabMAb products
PEG RabMAb ELISA Kit - New Complete Competition-based ELISA Kit
The PEG RabMAb ELISA kit (ab215546) is based on the competitive binding of the HRP-conjugated PEG and other molecules labeled with PEG for binding sites which are limited in number, on the surface of 96-wells in which Anti-PEG rabbit monoclonal antibody has already been coated.
The color development is then observed. The degree of coloration depends on the amount of competition between the enzyme-conjugated PEG and the substrate TMB. The color is inversely proportional to the number of PEGylated molecules in the sample, as shown in Figure 1.
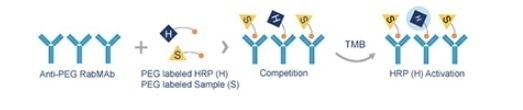
Figure 1. Competition between PEG labeled HRP (H) and PEG labeled sample (S) in PEG RabMAb ELISA assay
Polyethylene glycol (PEG) ELISA kit RabMAb (ab215546)
References
- Guiotto, A. et al. (2004), Anchimeric assistance effect on regioselective hydrolysis of branched PEGs: a mechanistic investigation ,Bioorg. Med.Chem. 12, 5031–5037.
- Wong, S.S. (1991), Reactive groups of proteins and their modifying agents. In Chemistry of Protein conjugation and cross-linking, p. 13, CRC Press.
- Caliceti, P. et al.(1993), Active site protection of proteolytic enzymes by poly(ethylene glycol) surface modification, J. Bioact. Comp. Polym. 8,41–50.
- Frank Leenders, celares GmbH, Berlin, Germany (2006), PEGylation technology and biopharmaceuticals. Biopharmaceuticals,39-40
 About Abcam
About Abcam
Abcam is a global life sciences company providing highly validated antibodies and other binders and assays to the research and clinical communities to help advance the understanding of biology and causes of disease.
Abcam’s mission is to serve life scientists to help them achieve their mission faster by listening to their needs, continuously innovating and improving and by giving them the tools, data and experience they want. Abcam’s ambition is to become the most influential life science company for researchers worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.