The divided solid materials have a significant share in the products produced or employed in the pharmaceutical industries (such as active pharmaceutical ingredient (API), excipients, additives, and so on).
Characterization of various physical behaviors of these materials is one of the greatest challenges of this century. Currently, the pressure is chiefly on the study of the relationships between the grain properties and the powders bed. Therefore, the aim is to control, analyze, and enhance the manufacturing processes of the powders.
One of the greatest benefits of the Dry Powder Inhaler (DPI) is that it enables better preservation of the formulation because of the absence of water in it. These inhalers are generally used to treat respiratory diseases such as bronchitis, asthma, emphysema, and obstructive chronic broncho-pneumopathy. Additionally, they enable the treatment of diabetes.
A majority of the capsules used in these inhalers include a micronized active drug (API) combined with coarse particles (excipients or carriers). The carriers avoid the segregation in the mix and offer a better flow capability to the formulation.
The aerosol generation is produced by the actuation of the particles from air introduced via several mechanisms of shearing and/or turbulence. Then, the powder bed enters into the lungs of the patient, where the drug particles are separated from the carrier prior to reaching most parts of the lungs.
For a majority of the DPI applications, the major challenge is optimizing the formulation to attain the largest FPF (fine particle fraction) while inhaling. For this, a vital parameter is to control the flow ability of the powder.
In fact, good flow properties enable the capsule filling to be kept regular as well as guaranteeing reproducible aerosol production, which ensures the amount of drug delivery to the patient. Therefore, the mixtures are characterized to measure cohesion, ventilation, flow ability, and compressibility of certain newly developed DPI formulations.
Materials and Methods
Four formulations were prepared in different ratios (ABL-20-2-5, ABL-20-2-10, ACL-20-2-20, and ABL-20-2-30), where each one consists of a similar excipient (Lactose: L) and two active drugs (A, B, or C).
After that, these combinations were characterized using new instruments (GranuDrum, GranuPack, and GranuHeap), particle size analyzer, and scanning electron microscopy.
This article discusses the relationships between packing measurement (GranuPack), rheological properties (GranuDrum), and grain size distributions. It also discusses the flow robustness powder.
Table 1. Pharmaceutical formulations prepared to contain a similar excipient (Lactose: L) and two active drugs (A, B, or C).
Results and Discussion
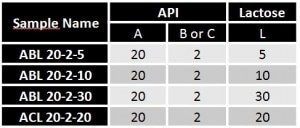
The comparison of the excipient grain size distribution (lactose — yellow discs on Figure 1) with the analysis of the grain size distributions of the blends reveals that the last mode is linked to the lactose grain particles. The first two modes represent the API’s subpopulations.
It is to be noted that the expansion of the area under the last mode is not surprising; the excipient volume fraction is reinforced as follows: ABL 20-2-5 < ABL 20-2-10 < ACL 20-2-20 < ABL 20-2-30.
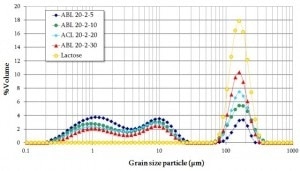
Figure 1. Dynamical tap test measurement for pharmaceutical blends—GranuPack.
Illustrated in Figure 1 is the dynamical tap test density measurement — the GranuPack — of the pharmaceutical blends. The ABL-20-2-30 formulation represents the weakest tap density variation of the series. It has the highest apparent density.
The ABL-20-2-5 blend exhibits the largest variation. It has the lowest initial density and the slowest packing/compressing kinetic (N1/2 = 15.0).
Unlike the curves of the samples ABL-20-2-30 and ABL-20-2-10, which appear linear (non-cohesive behavior), the curves of the samples ABL-20-2-5 and ACL-20-2-20 are sigmoid (cohesive behavior), indicating a particulate reorganization before being compressed.
The results of the analyses of the rheological dry powder measurement obtained from GranuDrum (see cohesive index curves on Figure 2) demonstrate that ABL-20-2-10 and ABL-20-2-30 blends are highly robust when compared to ACL-20-2-20 and ABL-20-2-5. Cohesive index measurements underline a discrepancy in the behavior of the front and back curves for the ACL-20-2-20 and ABL-20-2-5 blends.
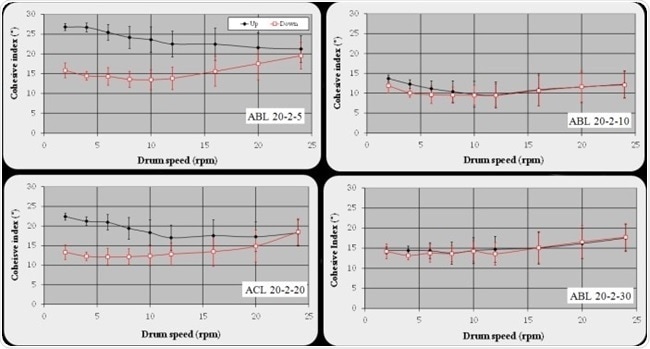
Figure 2. Flow robustness measurement of pharmaceutical blends for dry powder inhalers.
Of course, at lowest speeds, the back flow angles are weaker when compared to the front ones. It is necessary for these observations to be related to the existence of caked balls that are formed during the measurement process. These balls reduce the friction and agglomerates contact number and limit the granular system’s cohesion. The quantification of hysteresis of the cohesive index measurement offers information on the capability of the blends to retain their flow properties in a reversible manner.
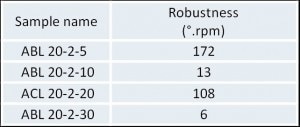
Table 2 provides the classification of the powder flow robustness (best results on the right): ABL-20-2-5 < ACL-20-2-20 < ABL-20-2-10 < ABL-20-2-30.
Table 2. Flow robustness measurement of pharmaceutical blends for dry powder inhalers
Conclusion
The measurements conducted on the formulations reveal that ABL-20-2-30 and ABL-20-2-10 blends exhibit the best flow properties. The ABL-20-2-5 formulation exhibits the poorest performances for capsules filling. The formulation ACL-20-2-20 lies in between these two ends.
The characterization of the rheological powder properties (flow, ventilation, compressing) provides several benefits. It guarantees a better quality control of the batches at different stages of their production. It saves time and money: the optimization of the new formulations is carried out instantly at the time of their development in the laboratory. When considering DPI applications, it is possible to correlate rheological properties and FPF.
About Granutools
GranuTools, is a company that improves powder understanding by delivering leading edge physical characterization tools.
“All we do is powder flow characterization”
A Set of Complementary Tools
Combining decades of experience in scientific instrumentation with fundamental research on powders characterization, we offer a unique set of complementary instruments for granular materials characterization.
Named after their purpose, our instruments are tools to understand macroscopic behavior of powders.
GranuFlow for flow, GranuHeap for static cohesion, GranuDrum for dynamic cohesion, GranuPack for tapped density and GranuCharge for triboelectric charge measurements are designed with the following in mind:
- Precise & Repeatable
- Automatic
- No Operator Dependency
- Robust & Easy to Use
- Clear Interpretation