I. Introduction
1. Generalities
There are a number of different steps involved in the tableting process of pharmaceutical powders. The most important of these is of course the filling of the die, and the compaction of the powder.
It is essential to have good flowing properties of the powders, in order to effectively guarantee a consistent flow through the die. In order to obtain low variability of the tablet mass and guarantee a uniform drug load to the patient, the filling of the die is of great importance.
Thanks to the fact that the drug powder typically has a flowability too low to be directly processed, the addition of specifically designed excipients is required to improve the flowability.
In order to help developing formulations with higher tableting performance, a better understanding of the influence of the drug load on the blend behavior is, therefore, a key parameter.
It is necessary to investigate the influence of the drug load on both the packing properties and flowability of pharmaceutical powders. Blends of Acetaminophen (APAP) and silicified microcrystalline cellulose (Prosolv SMCC90) have been produced at varying drug loads of APAP.
The first aspect to be investigated is the influence of the fraction of APAP on the packing dynamics and flowability of the blends, using the GranuPack and the GranuDrum.
Tablets are subsequently produced in a rotary press (Natoli® NP-RD30) in order to assess and evaluate the effect that modification of the powder behavior has on the mechanical properties of the tablets.
This investigatory process will illuminate how proper characterization of the powders can assist in the process of gathering essential information, in order to predict their performance in the tableting device.
2. GranuDrum
GranuDrum instrument takes the form of an automated powder flowability measurement method, which is based on the rotating drum principle. The sample of powder is used to half-full a horizontal cylinder with transparent sidewalls called drum. The drum rotates around its axis at an angular velocity which ranges from 2 rpm to 60 rpm.
During this process, snapshots are taken by a CCD camera (between 30 to 100 images separated by 1s) for each angular velocity. An edge detection algorithm is used to detect the air/powder interface is detected on each snapshot. Following this, the average interface position and the fluctuations around this average position are then computed.
Subsequently, the flowing angle for each rotating speed (which can also be referred to in relevant literature as ‘dynamic angle of repose’) αf is computed from the average interface position, and in addition the interface fluctuations are used to measure the dynamic cohesive index σf.
Generally speaking, a low value of the flowing angle σf corresponds to good flowability.
A wide set of parameters influence the flowing angle, such as: the shape of the grains, the friction between the grains, along with the cohesive forces (van der Waals, electrostatic and capillary forces) between the grains. The dynamic cohesive index σf is solely related to the cohesive forces between the grains.
A non-cohesive powder leads to a regular flow, while the inverse is true: a cohesive powder leads to an intermitted flow. Therefore, it can be concluded that a dynamic cohesive index close to zero corresponds to a non-cohesive powder. The cohesive index increases accordingly with the powder cohesiveness.
Figure 1. Sketch of GranuDrum measurement principle. Image Credit: Granutools
The GranuDrum allows the measurement of the first avalanche angle and the powder aeration during the flow, in addition to the measurement of both the cohesive index σf and the flowing angle αf as a function of the rotating speed. In this article, the powder rheology will be investigated with the cohesive index.
3. GranuPack
When it comes to powder characterization, the bulk density, the tapped density and the Hausner ratio measurement (commonly named 'tap-tap test') is very popular, thanks to the simplicity and the rapidity of the measurement.
When it comes to storage, transportation, caking and so on, the density and the ability of a powder to increase its density are key parameters. The pharmacopeia defines the recommended procedure. There are three major drawbacks to this simple test, which are outlined below.
The first of these drawbacks is that the result of the measurement depends on the operator; indeed, the initial powder volume is influenced by the filling method. The second is that a volume measurements reliant on naked eyes induces some significant errors on the results.
Thirdly, in this basic method, the compaction dynamics between the initial and the final measurements are missed.
Based on recent fundamental search results, GranuPack instrument is an automated and improved tapped density measurement method building on existing knowledge. An automatized device is used to analyze the behavior of the powder when the latter is submitted to successive taps.
Precise measurements are taken of both the initial density ρ(0) and the final density after n taps ρ(n), following which the Hausner ratio (Hr=ρ(n)/ ρ(0)) is then computed. The slope index α gives insight into the kinematics of the first stages of the packing, and is extracted from the packing curves.
The higher the slope index is, the slower the packing is. The Hausner ratio and the slope index will be sued in this investigation, in order to investigate the modification of packing properties of the blends.
II. Powder description
Roller compacted Acetaminophen (APAP) drug has been blended with silicified microcrystalline cellule (Prosolv SMCC90) and 0.5% Magnesium stearate for this study. The mass fraction of APAP has here been varied from 0% (only Prosolv SMCC90 + 0.5% Mg Stearate) and 60 % in mass.
Investigation of the drug load on tablet properties has been undertaken using a Natoli® NP-RD30 rotary press configured at 25rpm.
III. Packing analysis
1. Experimental protocol
500 taps were applied to the sample with a taps frequency of 1Hz and the measurement cell free-fall was 1 mm (∝ tap energy) for each experiment with the GranuPack. Before each experiment, the powder mass was recorded.
During the process, a metallic tube was used to house the powder, following the rigorous automated initialization process of the GranuPack in order to remove user dependency. Following this, a light hollow cylinder was put on the top of the powder bed in order to keep the powder/air interface flat throughout the process of compaction.
The tube which contains the powder sample rose up to a fixed height of ΔZ and performed free falls. After each tap, the height h of the powder bed was automatically measured. The volume V of each pile was therefore computed from the height h. Given that the powder mass m is known, the bulk density ρ was evaluated and plotted after each of the taps.
2. Experimental results
Figure 2 depicts the complete packing curves obtained for APAP drug load, which range from 0% to 60% in mass. These results are also summarized and displayed in Table 1.
These Figures clearly evidence the differences in the packing curves, which indicates a significant influence of the drug on the packing properties of the blends. Increasing the drug load therefore leads to an increase of both the initial and the final bulk densities of the blends.
Figure 3 depicts the Hausner ratio evolution. This (the Hausner ratio) is typically used as a measure of the powder flowability. The typical relationship between the two is that the higher the Hausner ratio, the lower the flowability.
It seems that increasing the drug load thus leads to a significant increase in the Hausner ratio indicating a significant decrease in flowability, based on this measure. The blend without drug (%APAP) has an Hr=1.25 – so is therefore expected to display a good flowability.
In addition to this, the blend with 60% APAP displays a much higher Hausner ratio of 1.47 which indicates a significant increase of its cohesiveness.
Table 1. Initial (ρ(0)) and tapped (ρ(n)) bulk densities, Hausner ratio (Hr) and slope index measured with the GranuPack for the different drug loads. Source: Granutools
| Sample Name |
ρ(0) (g/ml) |
ρ(n) (g/ml) |
Hr |
Slope index |
| 0% APAP |
0,373 |
0,465 |
1,25 |
4,60E-03 |
| 20% APAP |
0,493 |
0,671 |
1,36 |
7,13E-03 |
| 40% APAP |
0,513 |
0,730 |
1,42 |
9,07E-03 |
| 60% APAP |
0,525 |
0,771 |
1,47 |
1,02E-02 |
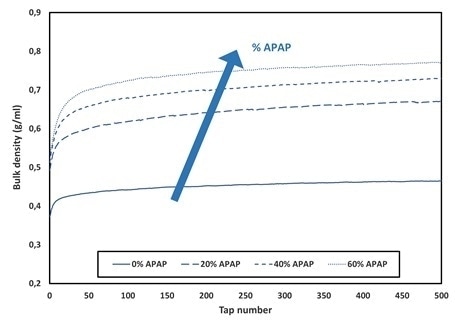
Figure 2. Packing curves obtained for the different drug load (%APAP). Image Credit: Granutools
Bulk density evolution is measured with the GranuTap after each tap, which allows investigation of the dynamics of packing. Figure 3 depicts the slope index, which is a measure of the packing kinematics.
Interestingly, despite the decrease of flowability predicted from the Hausner ratio analysis, it can be observed that the packing is faster for higher drug load.
Grains mobility and thus a slowdown of packing dynamics are expected to follow an increase of cohesiveness due to stronger cohesive interactions. In contract, an opposite behavior is indicated by the slope index analysis.
The powder is allowed to sustain a looser packing at rest thanks to the higher cohesiveness of blends with high drug load, giving room to quickly increase the bulk density after just a few taps.
In addition to this, the differences in particle sizes between the drug and the excipient could combine and lead to an increasing number of finer particles to the blend, with increasing drug load helping to quickly fill the gap between the larger grains during packing.
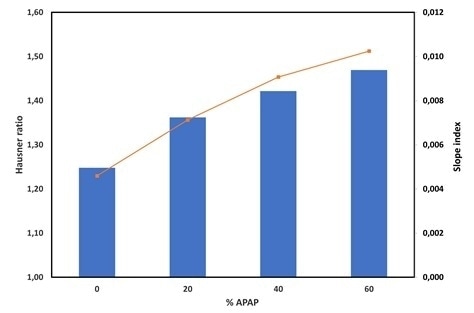
Figure 3. Hausner ratio (bars) and slope index (line) versus the drug load (%APAP). Image Credit: Granutools
IV. Flowability analysis
1. Experimental protocol
In the duration of an experiment with the GranuDrum, box opening occurred and immediately after, powders were poured inside the measurement cell. Approximately 50ml of powder was used.
Several GranuDrum velocities were investigated (ranging from 2 to 10 rpm). For this velocity, 40 pictures were taken, to facilitate the accuracy and repeatability of each of the measurements.
2. Experimental results
The cohesive measured at a rotating speed of 6 rpm obtained for the different drug load is depicted in Figure 4. A very good flowability is denoted by the blend with excipient solely (0% APAP) which exhibits the lowest cohesive index around 10.
A decrease of flowability of the blends is denoted by the fact that the increase of drug load is accompanied by an increase of cohesive index. This is in alignment with the packing analysis, which depicted an increase of Hr with drug load. The powder cohesiveness is increased by the drug load, thus inducing a decrease in its flowability.
In order to decrease the processing time, it is interesting to increase the tablet press speed. However, this approach requires the maintenance of a good flowability, in order to keep die filling consistent when the powder is submitted to a higher shear rate.
The cohesive index has been measured at different rotation speeds to investigate the rheology of the powder, i.e. its response in terms of cohesiveness due to an increasing applied stress. In Figure 5, the evolution of the cohesive index for speeds ranging from 2 to 10 rpm for the different drug loads is depicted.
The cohesive index exhibits a decrease of cohesive index with speed indicating a shear-thinning behavior for the excipient without drug (0% APAP). Tt is thus expected that the flowability is to be improved at higher shear. At the point of increasing the drug load, the rheological behavior is altered with the disappearance of the shear-thinning.
For high drug loads at higher tableting speeds, a decrease in tableting performance is thus expected. In particular, a variability may arise at high process speed of the mass of the tablets due to lack of consistency of the filling the die.
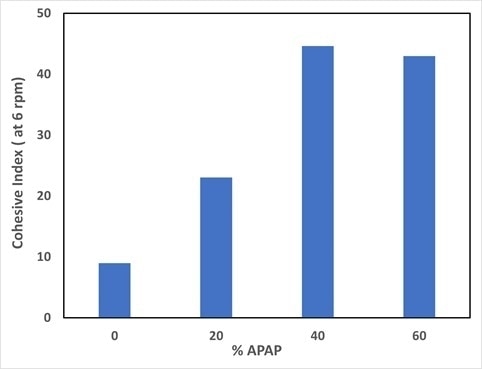
Figure 4. Cohesive index measured at a rotating speed of 6 rpm for the different drug load. Image Credit: Granutools
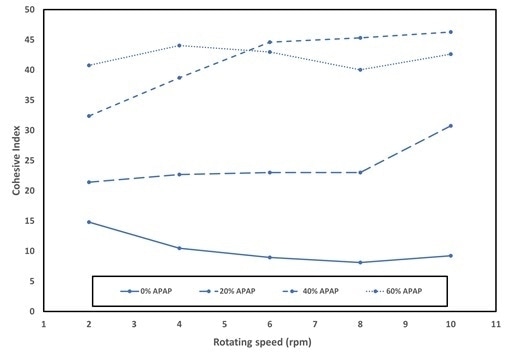
Figure 5. Cohesive index versus the rotating speed for drug load from 0% (only Provolv + Mg Stearate) and 60% APAP in mass. Image Credit: Granutools
V. Impact on tablet properties
1. Experimental protocol
The Natoli® NP-RD30 rotary press is set at 25 rpm to facilitate the investigation compression pressures of 50, 100, 150, 200, 250 and 300 Mpa. 10 tablets are produced for each compression pressure and the tensile strength for each is measured.
2. Experimental results
Figure 6 depicts the tablet tensile strength versus the drug load for the each of the different compression pressures investigated. The major decrease of tensile strength with increasing drug load clearly highlights the decrease of tablet quality.
It was not possible to produce tablets for drug load above 40% for the lowest compression pressure of 50 MPa. Higher tensile strength was facilitated by increasing the compression pressure; however, the decreasing trends with drug remains similar.
The addition of APAP impacts powder properties; thus significantly impacting the tablet quality. The drug load tends to decrease the flowability of the powder by increasing its cohesiveness, as shown by the blends packing and flowability characterization.
It thus appears that the blends cohesiveness has direct consequence on the tablet’s mechanical properties. Whatever the compression pressure used, the lower the flowability, the worse the tensile strength of the tablet.
The importance of a good powder characterization is thus highlighted by these results. Certainly, assessing the packing and flowing behavior of the blends beforehand will allow for the selection of suitable formulations, in order to produce tablets that meet the quality criteria and standards.
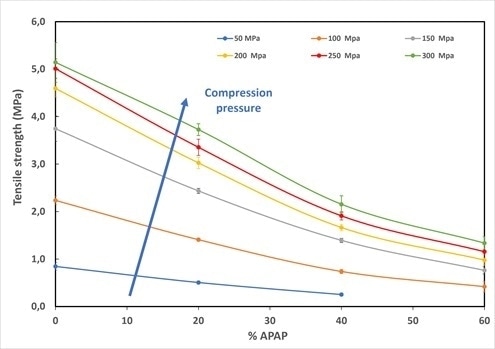
Figure 6. Tablet tensile strength obtained at different compression pressure as a function of the drug load (%APAP). Increasing the drug load leads to significant degradation of tablet mechanical properties. Image Credit: Granutools
VI. Conclusions
This article has investigated and analyzed the influence and impact of drug load on powder behavior. It has been found that the drug load induces a significant increase in powder cohesiveness. Investigation and analysis of packing using the GranuPack revealed an increase of Hausner ratio with drug load.
This finding denotes an increase of the global cohesiveness of the blends, as well as an influence on the packing dynamics of the powder.
Certainly, faster packing kinematics at high drug load were observed. This finding can be either attributed to the smaller initial bulk density or APAP’s effect of finer particles. The results obtained with the packing analysis were confirmed by flowability assessments performed with the GranuDrum.
A change in powder rheology have been evidenced, as a result of the multiple rotating speeds investigation. In accordance with this, the excipient without the drug (0% APAP) displays an advantageous shear-thinning behavior. This behavior then disappears at high drug load.
The powder characterization results have been analyzed and correlated with the actual mechanical properties of the tablet. The decrease of flowability at high drug load is accompanied by a significant decrease of tablet tensile strength, it has been revealed.
There is thus clear evidence for the importance of performing proper powder characterization, in order to improve blends formulation.
VII. Acknowledgments
These findings would not have been possible without Robert Sedlock, Kerry Cruz and Harsh Shah from Natoli, who were instrumental in taking the measurements and providing supporting data for the tablet performance study.
Bibliography
- Cascade of granular flows for characterizing segregation, G. Lumay, F. Boschin, R. Cloots, N. Vandewalle, Powder Technology 234, 32-36 (2013).
- Combined effect of moisture and electrostatic charges on powder flow, A. Rescaglio, J. Schockmel, N. Vandewalle and G. Lumay, EPJ Web of Conferences 140, 13009 (2017).
- Compaction dynamics of a magnetized powder, G. Lumay, S. Dorbolo and N. Vandewalle, Physical Review E 80, 041302 (2009).
- Compaction of anisotropic granular materials: Experiments and simulations, G. Lumay and N. Vandewalle, Physical Review E 70, 051314 (2004).
- Compaction Dynamics of Wet Granular Assemblies, J. E. Fiscina, G. Lumay, F. Ludewig and N. Vandewalle, Physical Review Letters 105, 048001 (2010).
- Effect of an electric field on an intermittent granular flow, E. Mersch, G. Lumay, F. Boschini, and N. Vandewalle, Physical Review E 81, 041309 (2010).
- Effect of relative air humidity on the flowability of lactose powders, G. Lumay, K. Traina, F. Boschini, V. Delaval, A. Rescaglio, R. Cloots and N. Vandewalle, Journal of Drug Delivery Science and Technology 35, 207-212 (2016).
- Experimental Study of Granular Compaction Dynamics at Different Scales: Grain Mobility, Hexagonal Domains, and Packing Fraction, G. Lumay and N. Vandewalle, Physical Review Letters 95, 028002 (2005).
- Flow abilities of powders and granular materials evidenced from dynamical tap density measurement, K. Traina, R. Cloots, S. Bontempi, G. Lumay, N. Vandewalle and F. Boschini, Powder Technology, 235, 842-852 (2013).
- Flow of magnetized grains in a rotating drum, G. Lumay and N. Vandewalle, Physical Review E 82, 040301(R) (2010).
- How tribo-electric charges modify powder flowability, A. Rescaglio, J. Schockmel, F. Francqui, N. Vandewalle, and G. Lumay, Annual Transactions of The Nordic Rheology Society 25, 17-21 (2016).
- Influence of cohesives forces on the macroscopic properties of granular assemblies, G. Lumay, J. Fiscina, F. Ludewig and N. Vandewalle, AIP Conference Proceedings 1542, 995 (2013).
- Linking compaction dynamics to the flow properties of powders, G. Lumay, N. Vandewalle, C. Bodson, L. Delattre and O. Gerasimov, Applied Physics Letters 89, 093505 (2006).
- Linking flowability and granulometry of lactose powders, F. Boschini, V. Delaval, K. Traina, N. Vandewalle, and G. Lumay, International Journal of Pharmaceutics 494, 312–320 (2015).
- Measuring the flowing properties of powders and grains, G. Lumay, F. Boschini, K. Traina, S. Bontempi, J.-C. Remy, R. Cloots, and N. Vandewalle, Powder Technology 224, 19-27 (2012).
- Motion of carbon nanotubes in a rotating drum: The dynamic angle of repose and a bed behavior diagram, S. L. Pirard, G. Lumay, N. Vandewalle, J-P. Pirard, Chemical Engineering Journal 146, 143-147 (2009).
- Mullite coatings on ceramic substrates: Stabilisation of Al2O3–SiO2 suspensions for spray drying of composite granules suitable for reactive plasma spraying, A. Schrijnemakers, S. André, G. Lumay, N. Vandewalle, F. Boschini, R. Cloots and B. Vertruyen, Journal of the European Ceramic Society 29, 2169–2175 (2009).
- Rheological behavior of β-Ti and NiTi powders produced by atomization for SLM production of open porous orthopedic implants, G. Yablokova, M. Speirs, J. Van Humbeeck, J.-P. Kruth, J. Schrooten, R. Cloots, F. Boschini, G. Lumay, J. Luyten, Powder Technology 283, 199–209 (2015).
- The flow rate of granular materials through an orifice, C. Mankoc, A. Janda, R. Arévalo, J. M. Pastor, I. Zuriguel, A. Garcimartín and D. Maza, Granular Matter 9, p407–414 (2007).
- The influence of grain shape, friction and cohesion on granular compaction dynamics, N. Vandewalle, G. Lumay, O. Gerasimov and F. Ludewig, The European Physical Journal E (2007).
About Granutools
GranuTools, is a company that improves powder understanding by delivering leading edge physical characterization tools.
“All we do is powder flow characterization”
A Set of Complementary Tools
Combining decades of experience in scientific instrumentation with fundamental research on powders characterization, we offer a unique set of complementary instruments for granular materials characterization.
Named after their purpose, our instruments are tools to understand macroscopic behavior of powders.
GranuFlow for flow, GranuHeap for static cohesion, GranuDrum for dynamic cohesion, GranuPack for tapped density and GranuCharge for triboelectric charge measurements are designed with the following in mind:
- Precise & Repeatable
- Automatic
- No Operator Dependency
- Robust & Easy to Use
- Clear Interpretation