Ewing's sarcoma (ES) affects over 200 children and adolescents annually in the United States.1 ES tumors exhibit high resistance to current therapies, and more than 70% of patients with metastatic ES tumors succumb to the disease.2 Even in localized ES cases, 30-40% of patients experience relapse, and the five-year survival rate following recurrence is 15-25%.3
A laboratory at the University of North Carolina required monoclonal populations of two Ewing's sarcoma cell lines, namely MHH-ES-1 and TC-71, that were resistant to two commonly used chemotherapeutic agents, SN-38 and etoposide, to investigate chemoresistance mechanisms. Traditional cloning methods proved ineffective in promoting cell proliferation.
Traditional cloning techniques, such as flow cytometers, droplet dispensers, and limiting dilution, are often time-consuming and labor-intensive, leaving individual cells isolated in wells devoid of growth factors and mitogens from neighboring cells.
CellRaft® technology provides an environment similar to flasks for cell culture, featuring spatial segregation. It also incorporates software-guided identification to locate CellRafts containing monoclonal colonies, facilitating their automated isolation into collection plates for subsequent expansion.
Methods
Image Credit: Cell Microsystems
Use of CellRaft Cytometry to identify rafts containing monoclonal colonies
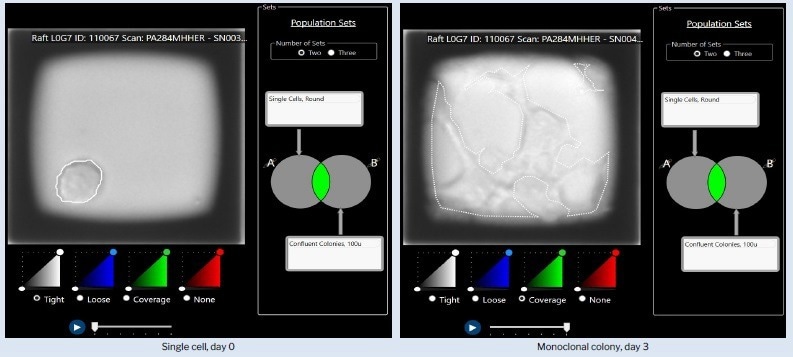
Figure1. Representative example of identification of monoclonal colonies using CellRaft Cytometry™. Two population sets, single cells on day 0 and confluent colonies on day 3, are overlayed to identify CellRafts containing monoclonal colonies. Image Credit: Cell Microsystems
Overlay of two sets of populations: single cells on day 0 and confluent colonies on day 3, facilitating the identification of CellRafts housing monoclonal colonies.
Over 1000 single cells were identified for each cell line
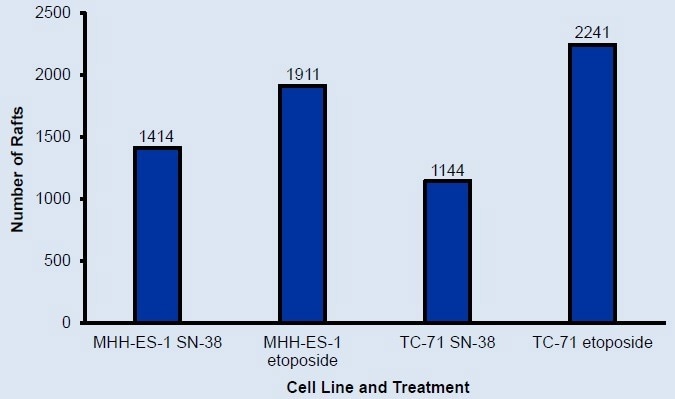
Figure 2. Number of rafts containing single cells at scan 1 identified using CellRaft Cytometry. Over 1000 single cells were identified for each cell line. Image Credit: Cell Microsystems
More than 1000 single cells were identified for each cell line.
Hundreds of monoclonal colonies grew for each cell line
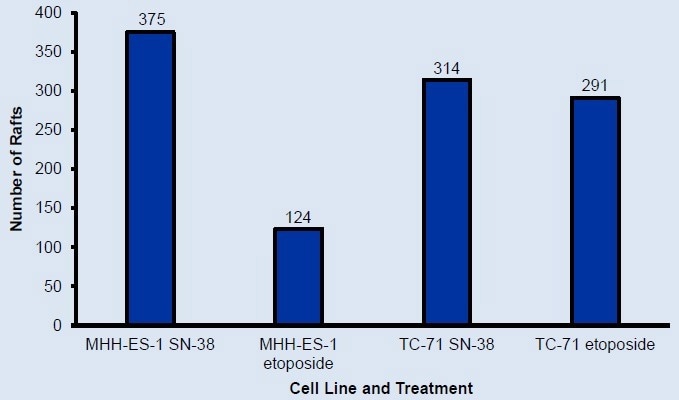
Figure 3. Number of rafts containing monoclonal colonies at final scan identified using CellRaft Cytometry. Over 100 monoclonal colonies for each cell line were available for isolation. Image Credit: Cell Microsystems
Isolation of over 100 monoclonal colonies was achieved for each cell line.
Successful expansion of monoclonal colonies for downstream testing
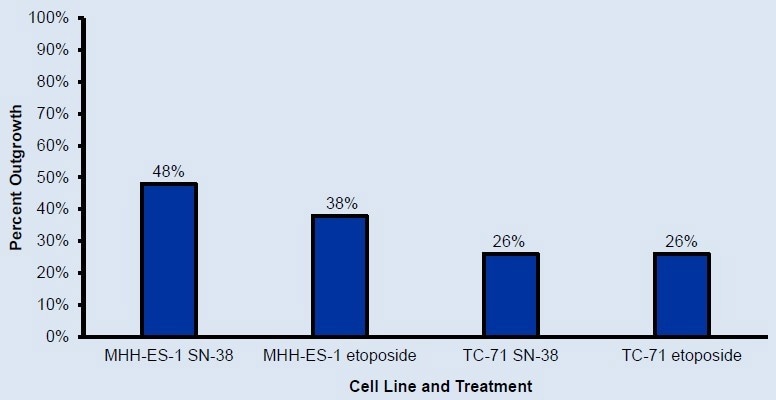
Figure 4. Percent of isolated rafts with growth off-raft after 7 days of incubation in collection plates. Colonies expanding off-raft can be incubated further until ready for expansion out of the collection plate. Image Credit: Cell Microsystems
Colony expansion off the CellRaft can be further incubated until ready for expansion outside the collection plate.
Visual confirmation of monoclonality and rapid colony growth
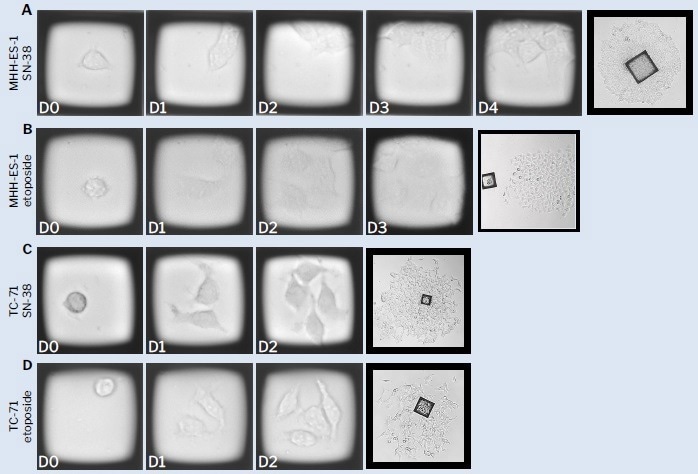
Figure 5. Representative time-course images of Ewing’s sarcoma cells on CellRafts. CellRaft arrays were imaged on the CellRaft AIR® system 4 hours post-seeding to identify single cells and every subsequent 24 hours to capture clonal growth. Monoclonal colonies were identified using CellRaft Cytometry. Growth off-raft was imaged on a bench microscope 5-10 days post-isolation. Image Credit: Cell Microsystems
CellRaft arrays were imaged on the CellRaft AIR® system four hours post-seeding to identify single cells and subsequently imaged every 24 hours to monitor clonal growth. CellRaft Cytometry was employed to identify monoclonal colonies. Growth outside the CellRaft was imaged using a bench microscope 5-10 days post-isolation.
Summary
Traditional limiting dilution failed to generate clones due to the inability of cells to proliferate in isolation. CellRaft technology generated more than 100 clones using one consumable per cell line.
Allowing single cells to proliferate within flask-like culture conditions with shared media significantly improved cloning efficiency compared to previous attempts using limiting dilution. Serial imaging of CellRafts provides a visual record of clonal growth and viability and doubling time assessments.
The entire workflow, from seeding the CellRaft Array to expansion outside the collection plate, can be completed in under three weeks with less than one hour of hands-on time, contrasting with over a year of unsuccessful attempts using limiting dilution.
CellRaft not only speeds up this process by providing many more clones, but more importantly, the unique monitoring process provided by CellRaft supplies additional information for each single clone with higher quality of clones.”
Associate Professor, University of North Carolina
References and further reading
- Esiashvili et al. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. (6):425-30 (2008).
- Balamuth & Womer. Ewing's sarcoma. Lancet Oncol. 11: 184–192 (2010).
- Bosma et al. Prognostic factors for survival in Ewing sarcoma: A systematic review. Surg Oncol. 27:603-610 (2018)
About Cell Microsystems
Making innovative tools that enable researchers to image, identify, and isolate viable single cells and clonal colonies
Researchers worldwide in the fields of CRISPR gene editing, oncology, stem cell biology, immunology and neurobiology use Cell Microsystems products, advancing sophisticated discovery across the life sciences.
The company’s CellRaft AIR® System addresses two widespread challenges facing scientists: the ability to actively select viable single cells or clonal colonies based on their phenotype, and match these cells to clonal expansion or molecular analyses. Cells are seeded, imaged, identified, and isolated on Cell Microsystem’s CellRaft® Arrays.
We have tested more than 100 Cell Lines with CellRaft Technology to demonstrate the high outgrowth efficiencies using our platform.
The company currently markets its products to researchers worldwide, and prides itself on being a customer-focused organization responsive to feedback and inspired to fuel deeper contributions to science.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.