Therapeutic proteins play a crucial role in the biological pharmaceutical sector, serving as treatment options for diseases such as diabetes, cancer, and anemia.
In recombinant protein development, a primary objective is establishing highly efficient monoclonal cell lines capable of producing substantial quantities of the desired protein. Chinese hamster ovary (CHO) cell lines have been the preferred commercial hosts for recombinant protein production, holding a prominent position in the industry.
Seeding a CellRaft Array Single Reservoir. Image Credit: Cell Microsystems
Achieving a homogeneous CHO cell line is not a straightforward task. Single-cell cloning is a regulatory requirement and a technical necessity in recombinant protein production due to the inherent variability in productivity among individual cells arising from the random integration of the transfected gene of interest.
The conventional approach to isolate and generate single cell-derived clones involves a limiting dilution method, which, unfortunately, suffers from low success rates, time constraints, and the need for extensive manual labor.
While various automated technologies, such as FACS, colony pickers, and droplet dispensers, have been developed to enhance the efficiency of a single cell or colony isolation, they often compromise cell viability due to the harsh manipulation and stress experienced by cells forced to grow independently in a large volume of media.
In contrast, the innovative CellRaft® Technology offers a streamlined and efficient solution for cell line development by combining the flask-like cell culture conditions of the CellRaft Array cell culture consumable with the automated isolation and imaging capabilities of the CellRaft AIR® System.
This CellRaft AIR System provides an automated alternative to the labor-intensive workflow, ensuring cell health, enabling time-course imaging for clonal verification, and facilitating automated isolation for downstream propagation. As a result, it presents a more time-efficient, cost-effective, and labor-saving approach to cell line development.
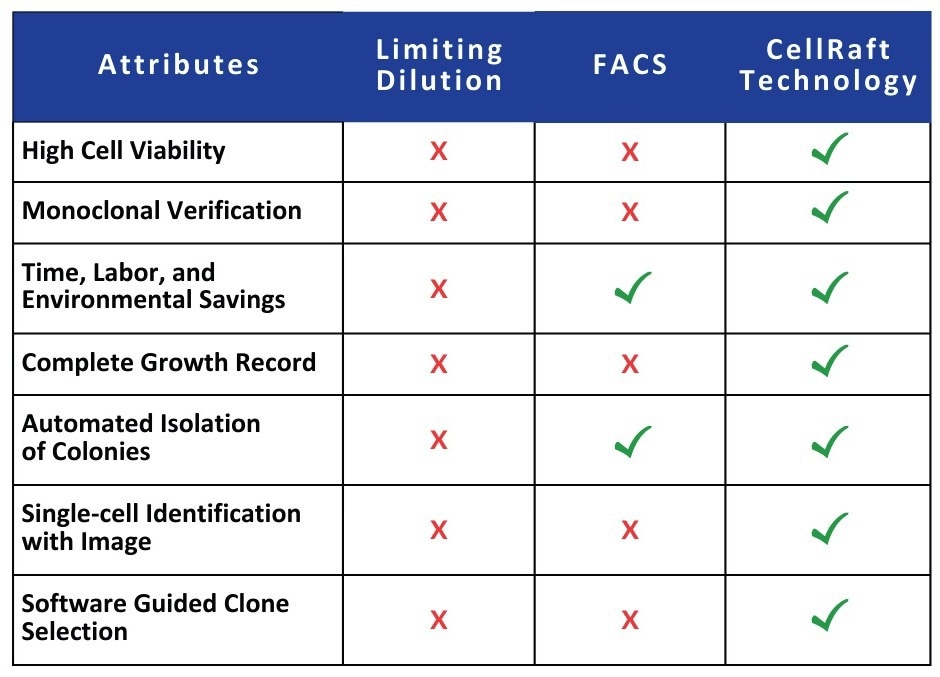
To highlight the value of CellRaft Technology in this context, a comparative analysis was conducted between the traditional limiting dilution method and the CellRaft Array using the CellRaft AIR System. The evaluation encompassed single-cell seeding efficiency, cloning efficiency, and clonal outgrowth.
Challenges and limitations with traditional methods of clonal cell line development
One of the significant obstacles in recombinant protein production is the development of monoclonal cell lines, which demands substantial investments of time and resources. The traditional method to achieve monoclonality is known as limiting dilution, whereby cells are diluted to a level with an approximate presence of one cell per well.
This approach proves to be time-consuming and requires a considerable amount of consumable resources. Plating cells at single-cell density often results in low efficiency, decreasing cell viability. (Figure 1, Table 1)
Due to the limitations of this technique, alternative technologies have emerged, such as cell sorting, automated clone picking, and single-cell dispensers. These aim to enhance the selection, delivery, and recovery of single cells but involve extensive manipulation of cells and plating methods that can harm cell viability.
There remains a pressing need for an automated system that preserves cell viability and efficiently generates monoclonal cell lines while remaining affordable for academic institutions and small industry laboratories, improving overall throughput.
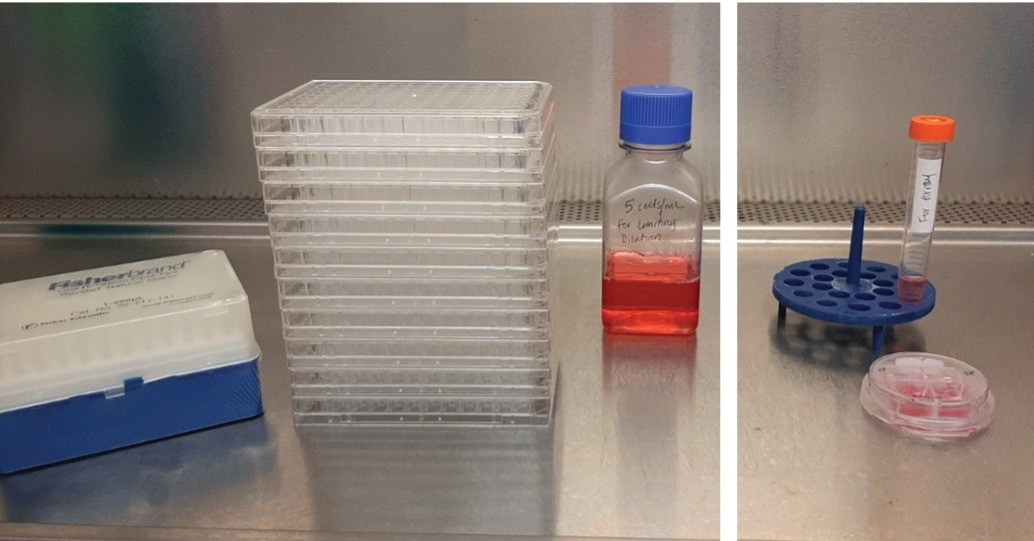
Figure 1. Ineffi cient limiting dilution workfl ows (left) require signifi cantly more consumables and media to generate monoclonal cell lines compared to the CellRaft Array (right) when seeding equal total cell counts. Image Credit: Cell Microsystems
Table 1. Comparing limiting dilution and FACS to the CellRaft Technology. Source: Cell Microsystems
Monoclonal cell line development using CellRaft technology
The generation of monoclonal cell lines can be effectively accomplished using the innovative CellRaft AIR System in conjunction with the CellRaft Array. The initial step involves seeding cells onto the CellRaft Array, a disposable microwave substrate for culturing and imaging purposes.
As the cells settle and adhere to the polystyrene CellRafts at the bottom of each microwell, they share a contiguous media reservoir.
This unique feature enables the exchange of growth factors and other cell secretions, ultimately improving cell viability and proliferation. Notably, this advantage is absent in other single-cell plating methods like limiting dilution, FACS, and droplet dispensers.
Following cell adherence, the CellRaft Array is scanned and imaged using the CellRaft AIR System, requiring only 10 minutes. Initially, the scanning process identifies CellRafts housing a single cell, and subsequently, periodic imaging is conducted to monitor the formation of colonies on the array originating from those single cells. (Figure 2)
The CellRaft AIR System comprises an optical system capable of performing bright field and three-channel fluorescent imaging. Conveniently, the software stores the scans in a centralized file for each array, enabling the analysis of phenotypic characteristics of cells of interest and facilitating the tracking of individual CellRafts and colony growth over time.
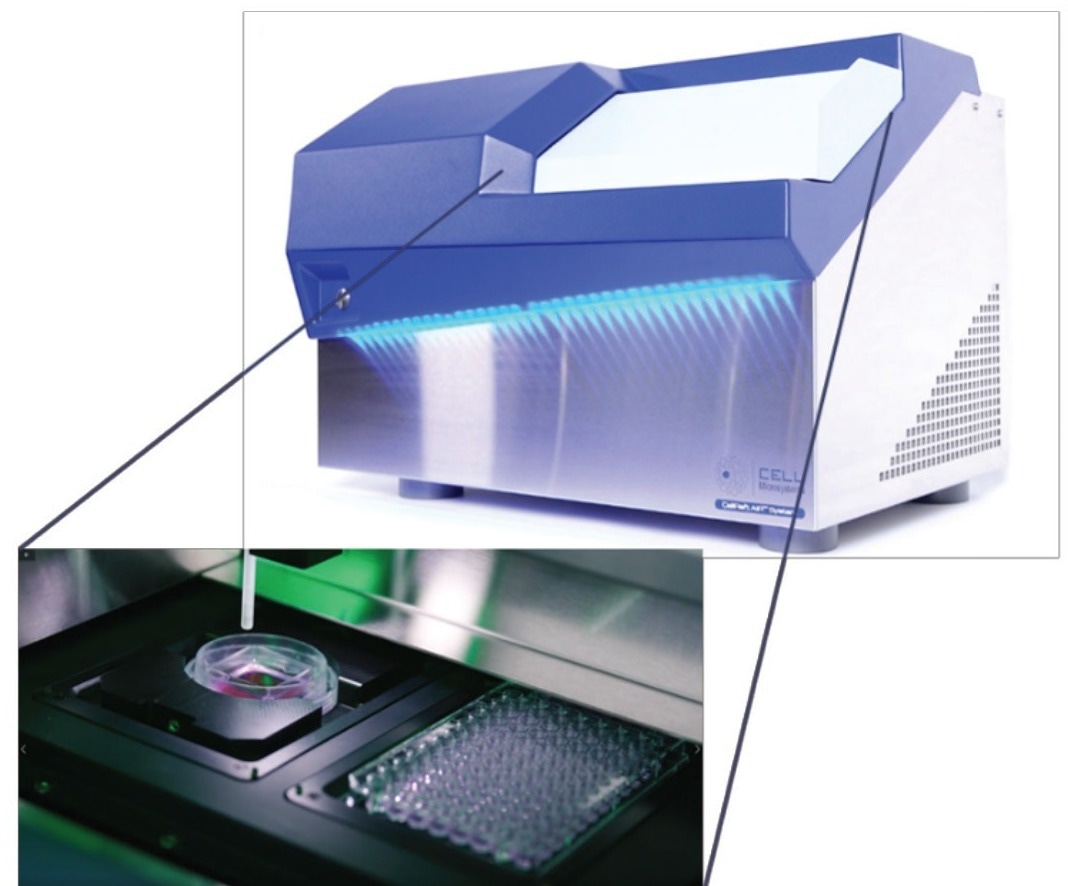
Figure 2. The CellRaft AIR System and CellRaft Array provide fully integrated brightfi eld and 3-channel fl uorescent imaging, automated release and transfer, and optional stage-top incubation. Image Credit: Cell Microsystems
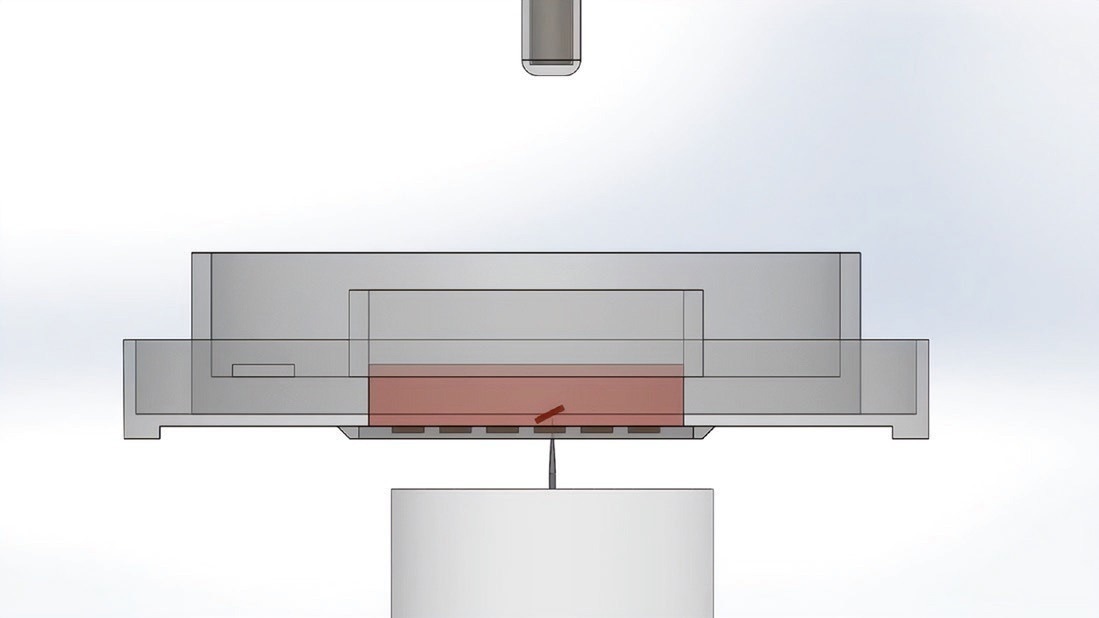
Once CellRafts housing monoclonal colonies are identified, they can be selectively released from the CellRaft Array and transferred into a 96-well plate for further expansion. This is done by using a motorized needle that penetrates the resealable elastomeric substrate of the CellRaft Array, dislodging the individual CellRaft from its microwell.
The CellRaft material is loaded with magnetic nanoparticles, allowing for retrieval using a magnetic wand. The cells remain attached to the CellRaft throughout the transfer process, obviating trypsin or other dissociation reagents. This preservation of cell viability and phenotype, coupled with isolation at the small-colony stage, proves advantageous.
Overall, this workflow significantly reduces hands-on time and substantially enhances output, presenting a more time-efficient, labor-saving, and cost-effective alternative for cell line development.
The desired microwell with cells is dislodged from the array. Image Credit: Cell Microsystems
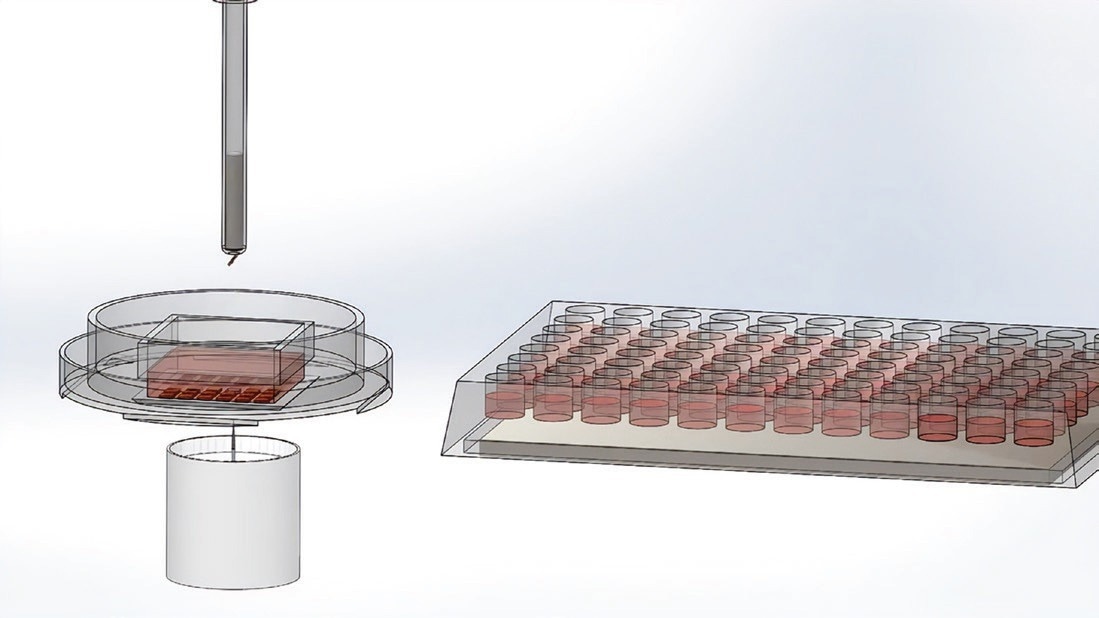
The wand picks up the microwell using a magnet. Image Credit: Cell Microsystems
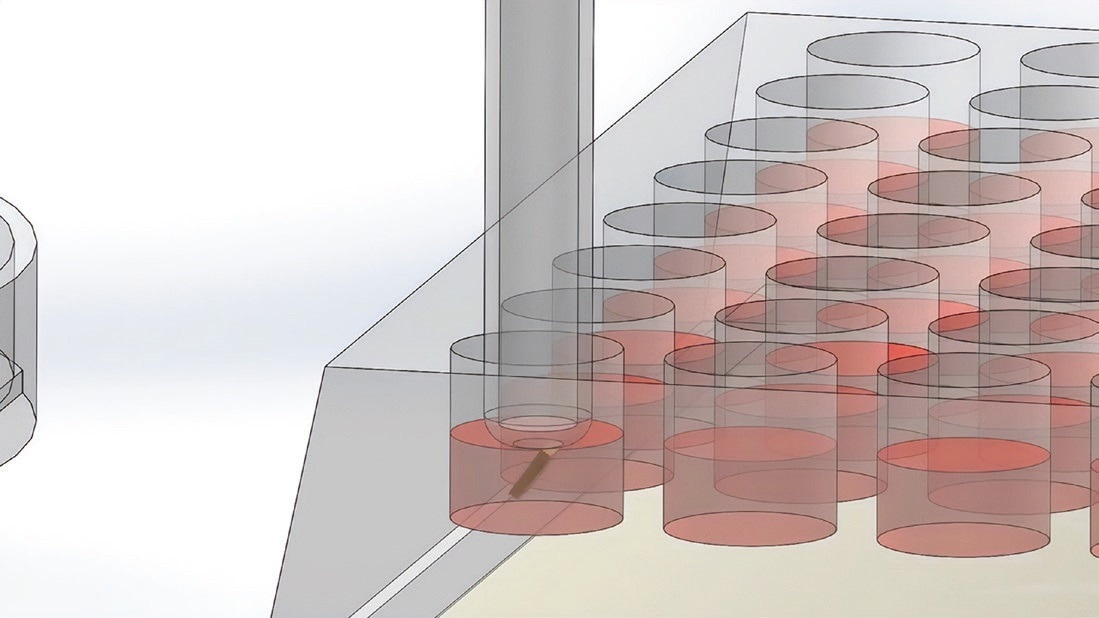
The wand places the microwell with the cells in the 96-well plate. Image Credit: Cell Microsystems
Comparing limiting dilution and CellRaft Array workflows
To demonstrate the user-friendly workflow, efficacy, and maintenance of high cell viability offered by the CellRaft Technology in single-cell clonal expansion workflows, a direct comparison of cell seeding efficiency and clonal outgrowth between the CellRaft Array and CellRaft AIR and limiting dilution methods was conducted, using widely utilized CHO-K1 cells in biopharmaceutical applications.
Two variants of the single-reservoir CellRaft Array were assessed: with 100x100 μm (100S) CellRafts and a 200x200 μm (200S) CellRafts, to investigate whether the size of the CellRaft and the resulting colony would have any impact on the outgrowth of colonies after isolation and transfer to a 96-well plate.
For each format (Limiting Dilution, 100S, and 200S), three 96-well plates were assessed for colony outgrowth. The workflow for each method is outlined below. For limiting dilution, 2,000 cells were seeded in well A1 of each 96-well plate, followed by a two-series serial dilution.
Starting from well A1, cells were serially diluted (1:2) down the first column and subsequently across each row of the plate (Figure 3).
Plates were incubated at 37 °C with 5% CO2 and monitored for clonal outgrowth. In the case of cell seeding on the CellRaft Arrays, two arrays were prepared: one with 100S CellRafts and another with 200S CellRafts. These arrays were seeded with 20,000 cells and 5,000 cells, respectively, using standard procedures (CellRaft Array user guide).
After allowing the cells to settle into the microwells and adhere to the CellRafts, each array was scanned using the CellRaft AIR System to identify CellRafts containing a single cell.
Full array scans were performed every 24 hours to monitor the clonal propagation of single-cell CellRafts until they were isolated and transferred to three 96-well plates per array.
Selected clones were isolated from the 100S array 48 hours after seeding, while clones from the 200S array were isolated after 72 hours to ensure complete colony coverage on the larger CellRafts.
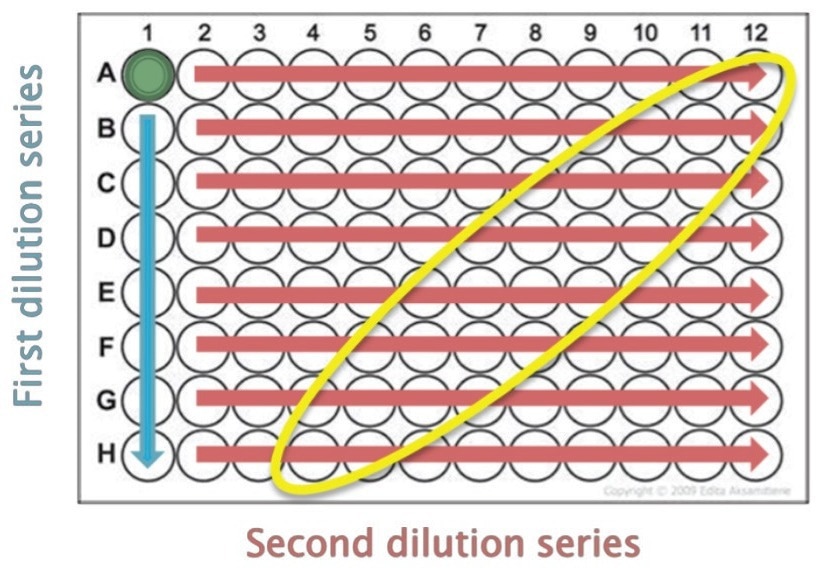
Figure 3. Two-series serial dilution for limiting dilution plates usedto generate single cells/well. The region of expected single cell clones is identifi ed in yellow. Image Credit: Cell Microsystems
Monitoring clonality on the CellRaft AIR
The crucial aspect of cell line development lies in the propagation of cells from a single cell. The CellRaft workflow outperforms traditional limiting dilution methods by offering visual confirmation of single cells and convenient monitoring of thousands of cells on a single consumable.
Using the CellRaft AIR System, all CellRafts present on each array were imaged at a resolution that allowed tracking from a single cell to a small colony. The data was stored in a centralized database, enabling the visualization of individual CellRafts over time. (Figure 4) Furthermore, the viability of the cells was monitored throughout the week.
In contrast, limiting dilution necessitates the seeding and manipulation of numerous 96-well plates, making it challenging to confirm the deposition of a single cell and verify clonality without specialized imaging equipment or scanners.
Leveraging the proprietary software of the CellRaft AIR System, we successfully identified over 5,400 single cell-derived clones on the 100S array and 1,100 single cell-derived clones on the 200S array, (Figure 5) all available for isolation into 96-well plates.
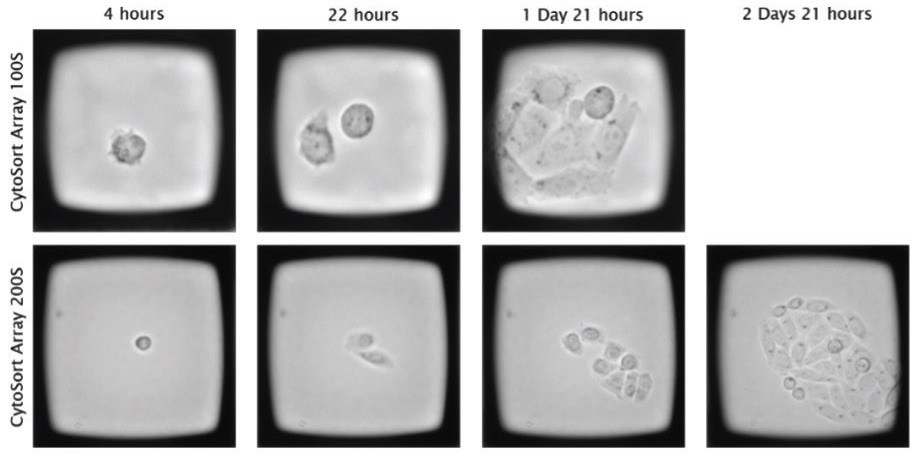
Figure 4. CHO cells seeded on the CellRaft Array are imaged and traced over time to monitor clonal outgrowth prior to isolation. Image Credit: Cell Microsystems
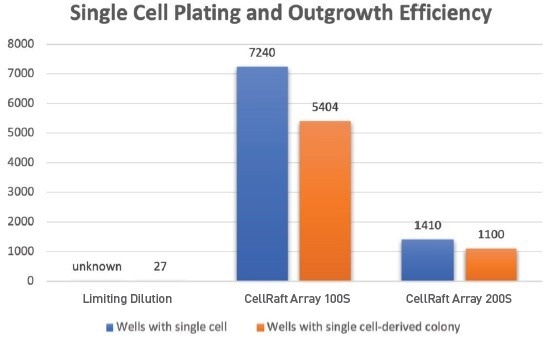
Figure 5. Seeding CHO cells on the CellRaft Arrays results in thousands of microwells with a single cell that can be reliably imaged, tracked, and phenotypically characterized as they grow into clonal colonies ahead of isolation into 96-well plates for further expansion. Seeding n=3 limiting dilution plates revealed 27 wells with colony growth, which cannot be verifi ed as starting from a single cell. Image Credit: Cell Microsystems
Clonal outgrowth using limiting dilution versus the CellRaft AIR System
A total of 288 CellRafts with colonies derived from a single cell – which represent only 5% and 26% of all available clonal colonies on the 100S and 200S CellRaft Arrays – were isolated into three 96-well tissue culture plates for each array format.
Of the total wells populated from the 100S and 200S arrays, 256 (89%) and 275 (95%), respectively, exhibited continued outgrowth, (Figure 6) vastly outnumbering the 27 colonies (9.4% of total wells seeded) in the three LD plates that had yet to be genotypically confirmed as clonal.
Based on these numbers, using limiting dilution for single-cell cloning would require the order of 30 96-well plates to achieve the equivalent number of clones as populating three 96-well plates with the CellRaft AIR System, resulting in a ten-fold reduction in plastic ware.
If more clones were necessary for evaluation, hundreds of single cell-derived colonies were still available and growing on the CellRaft Arrays.
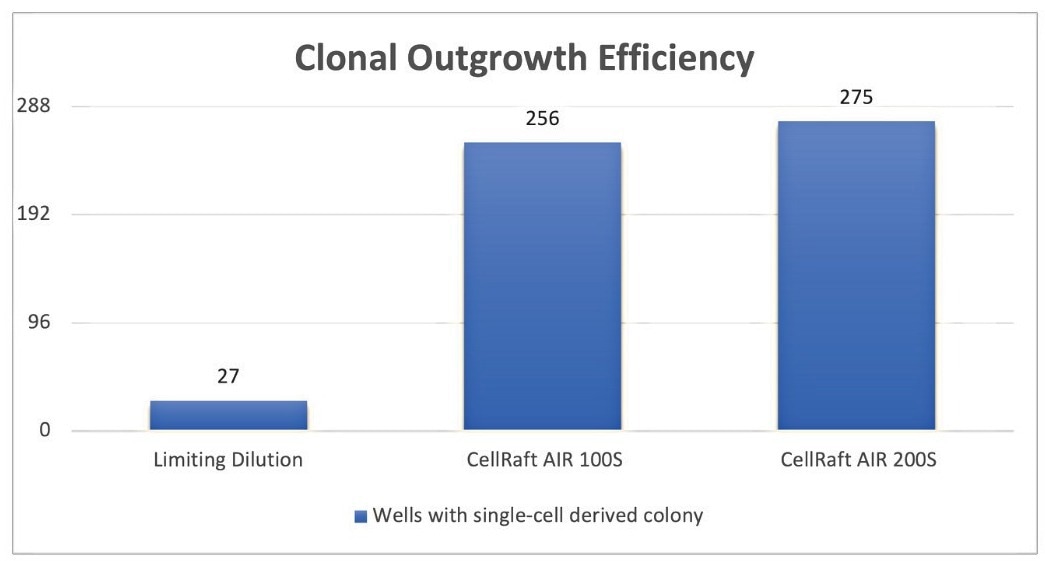
Figure 6. Clonal outgrowth effi ciency of CHO-K1 cells in n=3 96-well plates using Limiting Dilution, CellRaft AIR 100S and 200S formats. Image Credit: Cell Microsystems
Seven days post-isolation into 96-well plates, colonies were dissociated for expansion using 0.25% trypsinEDTA to disperse cells in the 96-well plates. (Figure 7)
After an additional three days of colony growth, wells were stained with Crystal Violet, which identifies viable cells and colonies, to visually demonstrate the improved cloning efficiency of the CellRaft AIR System compared to Limiting Dilution (Figure 8).
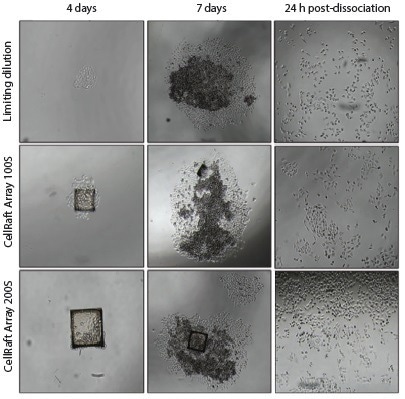
Figure 7. Representative images of CHO-K1 colony formation usingLimiting Dilution, 100S, and 200S cell-seeding formats in 96-well plates 4 days and 7 days after initial cell seeding and 24-hours after dissociating colonies using 0.25% trypsin-EDTA. Image Credit: Cell Microsystems
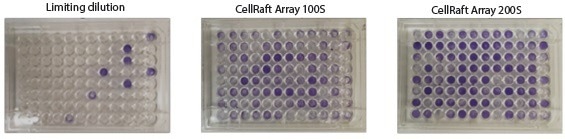
Figure 8. Representative 96-well plates of single cell derived CHO-K1 clones stained with Crystal Violet 10 days postseeding. Image Credit: Cell Microsystems
Conclusions
The CellRaft AIR System and CellRaft Array demonstrated superior performance compared to limiting dilution in the development of monoclonal CHO-K1 cell lines.
The technology provides an automated solution for a typically labor-intensive workflow, incorporating a specially designed consumable that promotes cell health.
It enables high-resolution imaging to verify clonality at the single-cell stage, tracks the growth of small colonies through time-course imaging, and automates the isolation process for subsequent propagation.
By adopting this technology for monoclonal cell line development methodologies, your laboratory can expedite the selection of the most suitable clone for your specific application. This transition reduces the overall effort, cost, and waste involved and enhances operational efficiency and success rates.
About Cell Microsystems
Making innovative tools that enable researchers to image, identify, and isolate viable single cells and clonal colonies
Researchers worldwide in the fields of CRISPR gene editing, oncology, stem cell biology, immunology and neurobiology use Cell Microsystems products, advancing sophisticated discovery across the life sciences.
The company’s CellRaft AIR® System addresses two widespread challenges facing scientists: the ability to actively select viable single cells or clonal colonies based on their phenotype, and match these cells to clonal expansion or molecular analyses. Cells are seeded, imaged, identified, and isolated on Cell Microsystem’s CellRaft® Arrays.
We have tested more than 100 Cell Lines with CellRaft Technology to demonstrate the high outgrowth efficiencies using our platform.
The company currently markets its products to researchers worldwide, and prides itself on being a customer-focused organization responsive to feedback and inspired to fuel deeper contributions to science.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.