Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), genome engineering of cell lines, facilitate target discovery. It provides an excellent disease model for preclinical research, particularly in chimeric antigen receptor T-cell therapy.
The primary challenge in cell engineering remains the generation of single-cell clones.
Current approaches range from manual limiting dilution (preferred for adherent lines) to a combination of high-speed cell sorters and bulk sorting followed by plating into single cells.
Objective
To enhance the frequency of single-cell clone generation, the researchers explored various available technologies, including different types of cell sorters and an image-based cell isolation system called CellRaft AIR™ developed by Cell Microsystems.
Methods
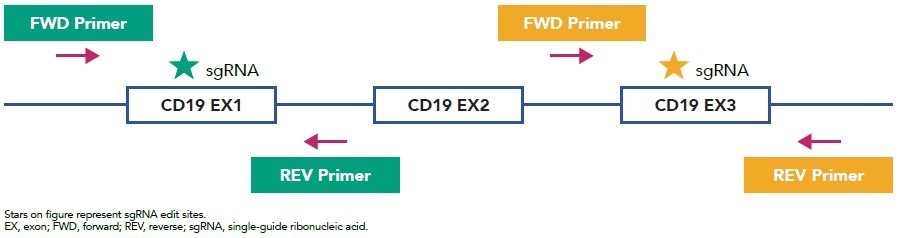
Figure 1. sgRNA and Primer Design. Image Credit: Cell Microsystems
- The gene of interest was targeted by designing specific single guide ribonucleic acids (sgRNAs) and primer sets (Figure 1).
Table 1. CD19 sgRNAs Primer Sequences.
| CD19 sgRNA |
Design ID from IDT |
Primers Flanking sgRNA Region |
| EX1: 5'CTCGGGCCTGACTTCCATGG3' |
Design ID:
CD.Cas9.FSVH8562.AO |
FWD: Primer 1
REV: Primer 1 |
| EX3: 5'CTAGGTCCGAAACATTCCAC3' |
Design ID:
Hs.Cas9.CD19.1.AA |
FWD: Primer 2
REV: Primer 2 |
EX, exon; FWD, forward; sgRNA, single guide ribonucleic acid; REV, reverse.
- Integrated DNA Technologies provided custom-designed guide RNAs based on their highest possible on/off target scores (Table 1).
- Primer sets were designed to amplify the target region surrounding the sgRNAs and to confirm indel formation through Sanger sequencing
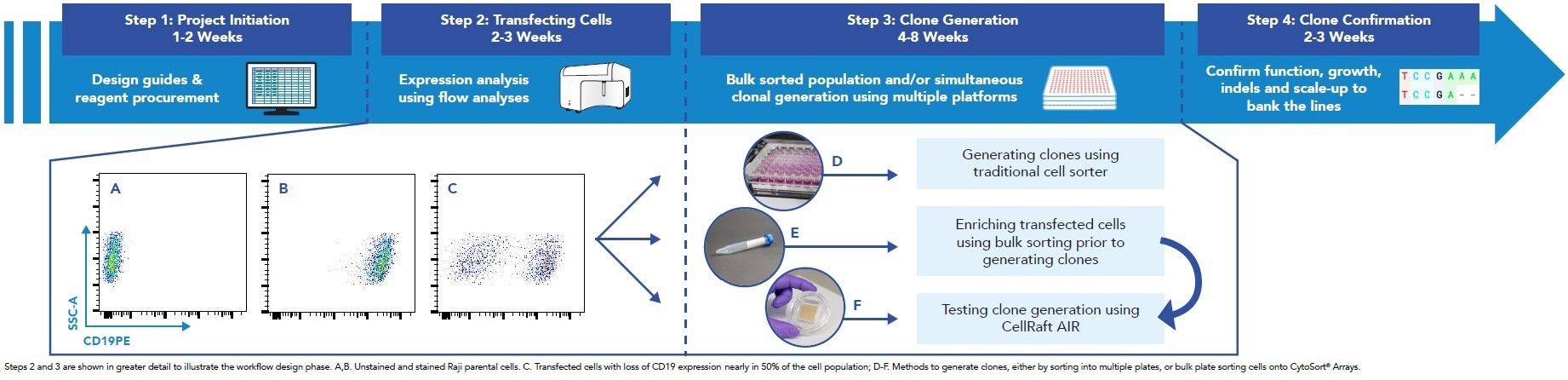
Figure 2. Timeline for Generating Engineered Cell Lines. Image Credit: Cell Microsystems
- The most time-consuming step in the process was generating clones from transfected or bulk-sorted cell lines (Figure 2)
- To streamline and improve efficiency, the researchers evaluated various cell sorters and Cell Microsystems' new product, the CellRaft AIR™
Table 2. Platforms Used for Single-Cell Clone Generation.
| |
 |
 |
 |
 |
| Objective |
BD FACS Aria Fusion |
SONY SH800 Sorter |
ThermoFisher Bigfoot Spectral Cell Sorter |
Cell Microsystems' CellRaft AIR Imaging and Cell Isolating System |
| Post sort viability (bulk) |
Almost 90% viable for suspension and 50% viable adherent lines |
Almost 90% viable for suspension and 50% viable adherent lines |
Almost 90% viable for suspension and adherent lines not tested |
Instrument not designed for bulk sort |
| Clone forming efficiency (per 96 well plate) |
Minimum 6-12 plates sorted, and 25-30 wells formed colonies (~30%) after 2-3 weeks based on cell type. Similarly, adherent cells plated and obtained on 3-5 clones from each plate (3-5%) |
Similar results observed from SONY sorter. Around 30% for suspension, 3-5% for adherent |
Similar results from Bigfoot sorter Around 30% for suspension, Adherent cells not tested |
Almost 400 clones form per array isolated into 3x96 well plates. Each plate will give rise to 50% single cells growing into colonies |
| Cell sort revival and colony growth |
3-4 weeks for suspension, 3-6 weeks for adherent |
3-4 weeks for suspension, 3-6 weeks for adherent |
3-4 weeks for suspension, adherent cells not tested |
3-4 weeks for suspension, adherent cells not tested |
| Instrument's ease of use (start-up, shut down times, and maintenance) |
40-60 minutes instrument start-up time (stabilizing stream based on nozzle used). Shut down takes 15 minutes |
20-minute instrument start-up time (stabilizing stream based on nozzle used). Shutdown takes 5-10 minutes; however, needs regular overnight shutdowns every month |
15-minute instrument start-up time (stabilizing stream based on nozzle used). Manufacturers still optimizing the system to make it easier for users to work with |
User-friendly imaging system works by simply switching on to scan, monitor cell growth, select better clones, and isolate (transfer) into 96 well plates |
- A comparison was made between the conventional sorters (BDFACSAria, SONY SH8000, and Thermofisher Bigfoot) and the imaging-based single-cell isolation system, Cell Microsystems' CellRaft AIR™, using several parameters such as ease of use, start-up time, and shut-down processing times (Table 2)
Results
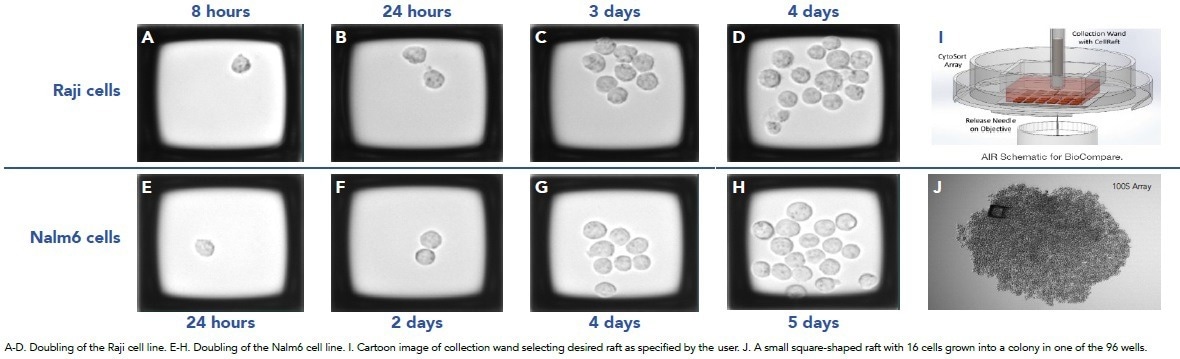
Figure 3. Images from Cell Microsytems CellRaft AIR™. Image Credit: Cell Microsystems
- The CellRaft AIR™ images demonstrate a doubling in the Raji cell line (Figures 3A-D) compared to the Nalm6 cell line (Figures 3E-J), showing sequential growth from a single cell to approximately the 16-cell stage
- The CellRaft AIR™ offers fluorescent imaging capabilities (not shown here) that aid in identifying specific populations based on fluorescent parameters
- The research team is currently exploring the use of this capability to transition directly from transfection to CellRaft AIR™ without the need for cell sorting
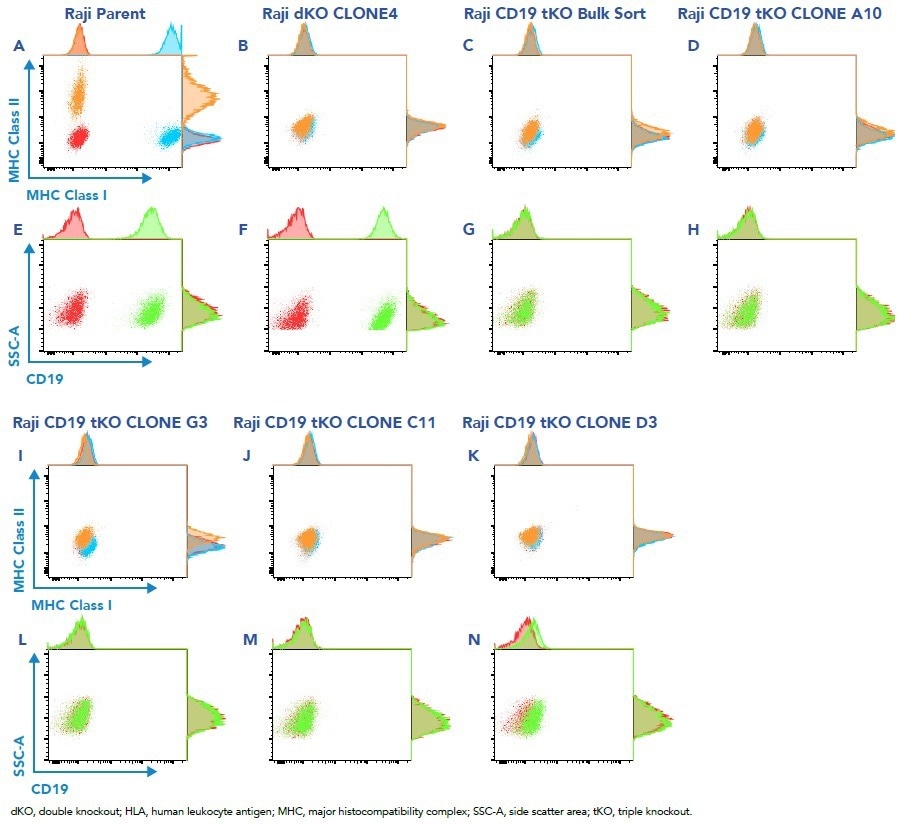
Figure 4. Bulk sort and clones analyzed for loss of protein expression by flow cytometry. Image Credit: Cell Microsystems
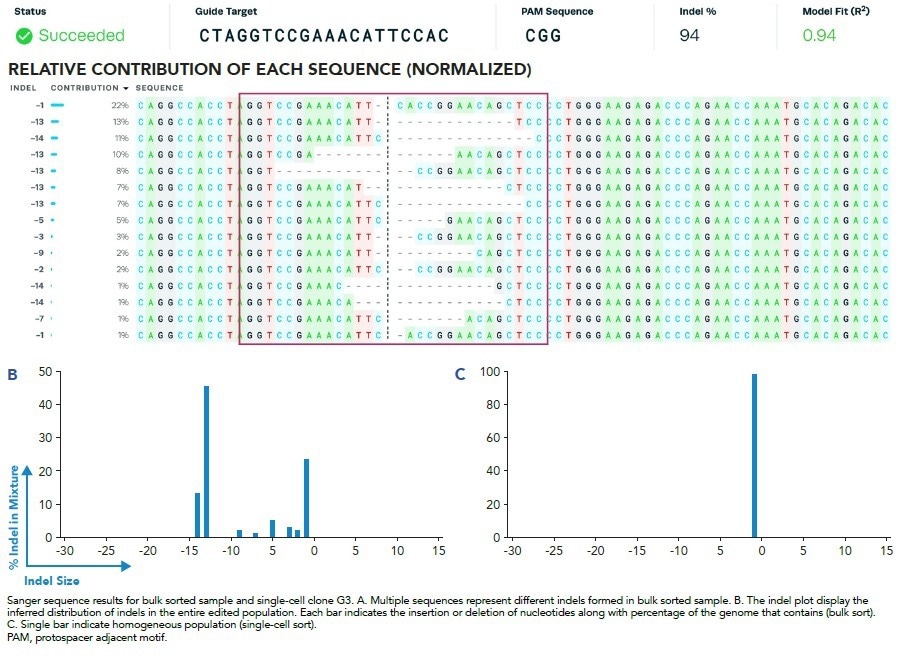
Figure 5. Indel Formation and Analysis. Image Credit: Cell Microsystems
- The wild-type parent Raji cells exhibit high expression of major histocompatibility (MHC) human leukocyte antigen Class I, Class II, and CD19 genes (Figures 4A, 4E)
- The double knockout clone 4 demonstrates the loss of MHC Class I and II (Figure 4B) and high expression before infection with CD19 sgRNA (Figure 4F)
- Bulk-sorted cells display no expression of MHC Class I/II or CD19 (Figures 4C, 4G)
- Clones derived from the bulk (A10, G3, C11, D3) show complete loss of expression of MHC Class I/II (Figures 4D, 4I, 4K) and CD19 (Figures 4H, 4L, 4N)
Conclusions
The observation reveals that the outgrowth of single cells from 96-well plates sorted using BDFACSAria, SonySH8000, and Thermofisher's Bigfoot sorters is limited to 30% or less.
Leveraging Cell Microsystems' CellRaft AIR™ imaging and isolation system, the researchers collected nearly 400 rafts containing single-cell-derived clones from each array. Over 60% of these clones exhibit potential for outgrowth. However, the kinetics and the number of clones required to be tested will vary based on cell type and transfection efficiency.
Several adherent cell lines tested in the laboratory do not withstand sorting at high pressure, and their outgrowth is poor when sorted individually in plates. Previously, manual limiting dilution was the only method to obtain clones. However, the CellRaft AIR™ system can also be utilized for adherent lines.
The CellRaft AIR™ system images and monitors cell growth from a single cell to at least 32 cells before transferring them into 96-well plates. Compared to other systems, better growth and survival rates of isolated cells were observed.
The instrument requires no start-up or shut-down time. This system streamlines the consolidation of clones from multiple plates, minimizing several steps.
The observed benefits of incorporating the CellRaft AIR™ system into the cell engineering workflow have resulted in shorter timelines and increased efficiency. To leverage all the features of CellRaft AIR™, the next steps involve staining and utilizing fluorescent parameters for clone selection.
There are plans to introduce automation for hit picking, PCR, and flow cytometry analyses to enhance timelines further.
Acknowledgments
The authors of the original poster are Sobha Thamminana, Aronpreet Atwell, Jessica Hartman, Richard Smith and Bhargavi Rajan.
Special thanks to our colleagues Marjorie Ison‑Dugenny, Martin Gomez, and Avery Jan Agustin for supporting this study.
Disclosures
ST: employment with, stock or other ownership in, and research funding from Kite, a Gilead Company. AA: employment with and research funding from Kite, a Gilead Company; stock or other ownership in Gilead. JH: no relevant financial relationships to disclose. RS: employment with and travel support from Kite, a Gilead Company; stock or other ownership in and patents from Amgen; stock or other ownership in Gilead. BR: employment with, stock or other ownership in, and research funding from Kite, a Gilead Company
About Cell Microsystems
Making innovative tools that enable researchers to image, identify, and isolate viable single cells and clonal colonies
Researchers worldwide in the fields of CRISPR gene editing, oncology, stem cell biology, immunology and neurobiology use Cell Microsystems products, advancing sophisticated discovery across the life sciences.
The company’s CellRaft AIR® System addresses two widespread challenges facing scientists: the ability to actively select viable single cells or clonal colonies based on their phenotype, and match these cells to clonal expansion or molecular analyses. Cells are seeded, imaged, identified, and isolated on Cell Microsystem’s CellRaft® Arrays.
We have tested more than 100 Cell Lines with CellRaft Technology to demonstrate the high outgrowth efficiencies using our platform.
The company currently markets its products to researchers worldwide, and prides itself on being a customer-focused organization responsive to feedback and inspired to fuel deeper contributions to science.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.