The progress in induced pluripotent stem cells (iPSCs) and organoid technology has addressed the lack of suitable in vitro models for human neurodevelopmental and degenerative disorders.
Over the past decade, various protocols and commercial kits have emerged, facilitating the differentiation of iPSCs into multicellular neuronal organoids that closely mimic the developmental processes and functional physiology of the human brain, including specific cellular composition in different regions.
Despite these advancements, the widespread utilization of organoid models remains limited due to challenges related to reproducibility, scalability, and labor-intensive workflows.
To tackle these obstacles, the implementation of CellRaft® Technology is reported, which enables the development of streamlined and reproducible organoid workflows. This innovative approach offers reliable imaging, software-guided selection, and automated isolation of individual organoids for subsequent applications.
Key highlights
- Track and trace the growth and differentiation of hundreds of iPSC-derived organoids
- Fully automate the isolation of individual viable, intact organoids of interest
- Generate customized 96-well plates for organoid-based assays, such as clonal propagation, drug or toxicity screening, or target discovery
Traditional workflow
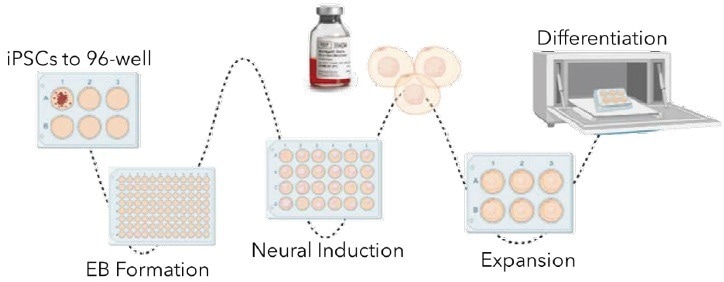
Image Credit: Cell Microsystems
Experimental design
Two edited iPSC lines, RFP or GFP, were cultured using standard methods in mTeSR Plus (StemCell Technologies). The iPSCs were then differentiated into cerebral and choroid plexus organoids using adapted protocols from commercially available kits (StemCell Technologies).
Initially, the iPSCs were dissociated into single cells or small fragments and seeded in a 1:1 ratio of GFP+ to RFP+ on the 500 µm CellRaft Array, suspended in an extracellular matrix in embryoid body formation media.
A CellRaft AIR® System performed an initial scan, capturing images of single or small cell fragments four hours after seeding.
The arrays were scanned every 24 hours to monitor organoid formation and differentiation. Media changes for neural induction and expansion were performed at the recommended time points by the manufacturer.
CellRaft Cytometry™ software identified CellRafts containing single iPSC-derived organoids based on their diameter (100-300 microns). These CellRafts with single organoids were isolated into a 96-well plate and cultured in cerebral maturation media starting from day 10.
The organoids were maintained in cerebral maturation media, with media changes every 2-3 days, until day 43. On day 28, non-GFP+ organoids were stained with NeuroFluor NeuO (StemCell Technologies) to confirm neural differentiation visually.
On day 43, single mature cerebral organoids were subjected to a six-point dose curve of ethanol (6.25-50 mM) for six hours to simulate acute alcohol exposure. The relative caspase activity was measured using the Caspase3/7-Glo Assay (Promega) to assess alcohol-induced apoptosis.
CellRaft technology workflow

Image Credit: Cell Microsystems
Results
Hundreds of iPSC-derived neuronal organoids were successfully generated and imaged on each array. The amended protocols allowed for establishing mono- and dual-fluorescent cerebral and choroid plexus organoids from single or multiple cells (Figure 1).
The CellRaft Cytometry software enabled the quantification of single-cell organoid forming efficiency across three replicate arrays for each organoid type (Figure 2). Clonal iPSC organoid formation exhibited a remarkable efficiency (> 17%).
Utilizing the AIR System, automated isolation of CellRafts containing single organoids was performed, depositing organoids selected based on a diameter range of 100-300 microns into 96-well collection plates.
This method preserved organoid morphology and viability, allowing subsequent off-array culture in cerebral maturation media up to day 42 (Figure 3). Remarkable three-dimensional growth and neural outgrowth (>98%), with positive staining for live neurons, were observed in cerebral organoids by day 25 (Figure 4A).
On day 40, images of fully mature cerebral organoids were captured. By selecting organoids for isolation based on the size on day 10, a 96-well plate with single mature organoids of relatively similar size was generated for downstream toxicity screening.
To evaluate the effect of acute alcohol exposure on mature cerebral organoids, an ethanol dose curve was conducted using day 43 cerebral organoids. At the highest doses, dose-dependent activation of caspase activity was observed, indicating alcohol-induced initiation of apoptosis (Figure 4C).
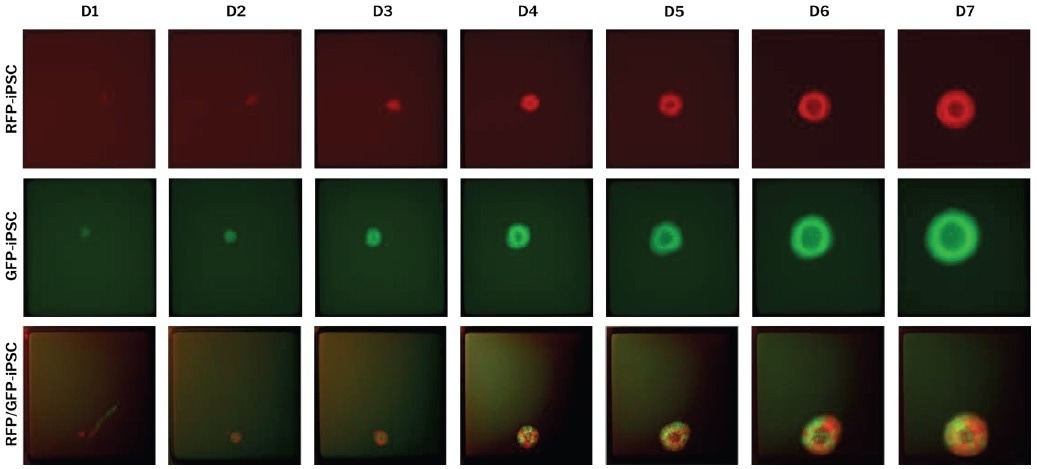
Figure 1. Edited single iPSCs are differentiated on the 500 μm CellRaft Array to form mono- and dual-fluorescent neural organoids. RFP- and GFP-edited iPSCs were co-cultured on the CellRaft Array in dilute ECM and imaged daily for a week during embryoid body formation and neural induction for choroid plexus organoid differentiation. Image Credit: Cell Microsystems
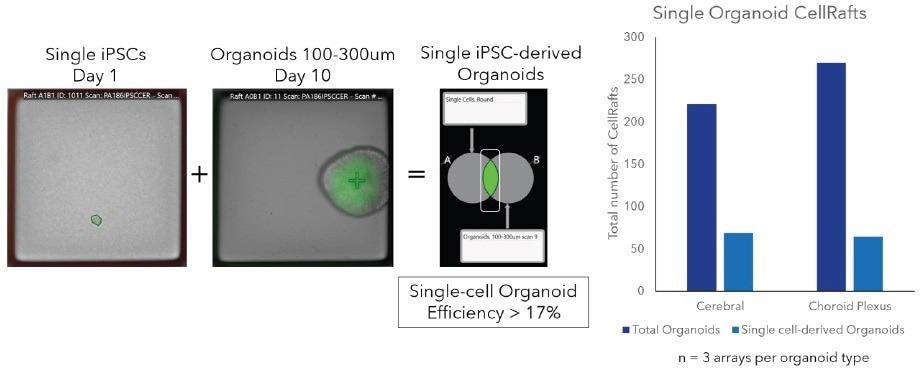
Figure 2. Image-based, software-guided organoid analysis. Using CellRaft Cytometry, user-defined populations can be built to identify CellRafts for isolation. For clonal workflows, populations identifying CellRafts with a single cell at day 1 and organoids at later timepoints can be overlayed to identify clonal organoids of interest, including fluorescence and morphological parameters. Image Credit: Cell Microsystems
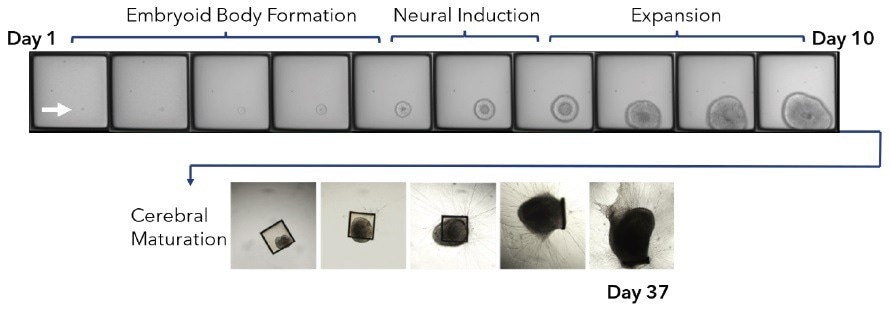
Figure 3. Clonal iPSC organoid neuronal differentiation. A mixed populations of edited (GFP or RFP) iPSCs were seeded on the 500 μm CellRaft Array in a suspension of Matrigel. The first several stages of organoid differentiation, embryoid body formation (days 1-5), neural induction (days 5-7), and expansion days (7-10), were performed on-array by performing media changes. The array was scanned every 24 hours to monitor organoid differentiation. At day 10, single organoids were isolated into 96-well plates into maturation media. Cerebral organoids were maintained in maturation media to day 42. Image Credit: Cell Microsystems
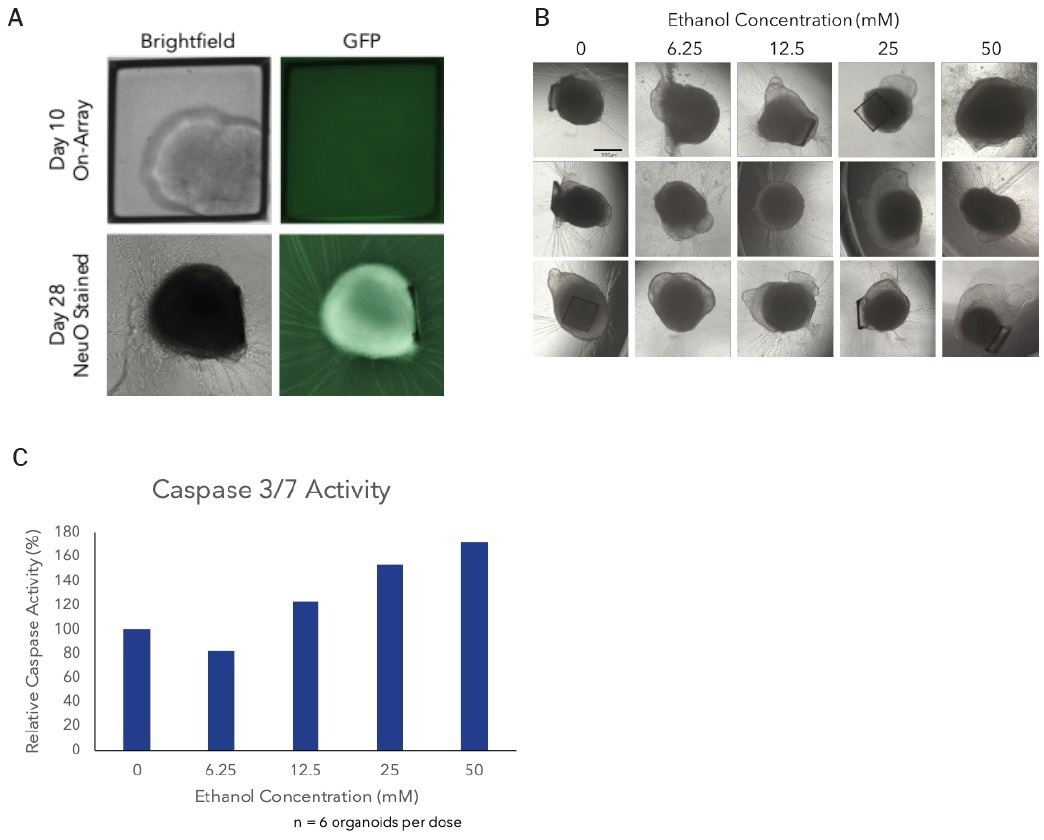
Figure 4. Alcohol induced apoptosis in neuronal organoids. On day 28, non-GFP organoids were stained using NeuroFluor NeuO, a membrane permeable fluorescent probe for detecting live neurons, as a visual confirmation of neural differentiation (A). Images of mature cerebral organoids on day 40, prior to treatment with ethanol, from three of the six replicate wells demonstrating the ability to generate well-to-well reproducibility it the downstream assay on day 40 by selecting organoids based on size at day 10 (B). On day 43, mature cerebral organoids were exposed to a 5-point dose curve of ethanol (6.25-50mM, n = 6 wells per dose) to simulate acute alcohol exposure. After 6 hours of treatment, organoids were evaluated for alcohol-induced apoptosis using the Caspase-Glo 3/7 Assay (Promega). We observed a dose-dependent increase in caspase activity, supporting activation of apoptosis at the highest doses of ethanol exposure (C). Image Credit: Cell Microsystems
Discussion
Utilizing the CellRaft technology, an efficient and user-friendly workflow has been demonstrated for cultivating and isolating numerous individual neuronal organoids derived from iPSCs.
The CellRaft Array serves as a distinctive platform for organoid culture, facilitating spatial segregation of cells and ensuring reliable imaging over time. This platform enables clonal organoid propagation, which is not feasible with conventional methodologies.
An exceptional attribute of the platform is CellRaft Cytometry, which provides a software-guided selection of single organoids contained within CellRafts, based on desired phenotypic, morphologic, and fluorescent properties. These selected organoids can then be isolated using the AIR System.
The data showcases how the CellRaft Technology addresses various challenges encountered in cerebral organoid workflows.
These include achieving higher yield and a less labor-intensive workflow, the ability to phenotypically characterize a heterogeneous organoid population, and the automated isolation of individual organoids of interest into 96-well plates for subsequent assays.
The CellRaft Technology unlocks the potential to generate customized 96-well plates of edited iPSC-derived organoids, which can be utilized in diverse downstream applications.
Conclusion
To expedite the integration of iPSC-derived organoids in pre-clinical applications, three primary challenges must be addressed: scalability, reproducibility, and implementing automated solutions to streamline otherwise manual workflows.
Employing the CellRaft Array makes it feasible to cultivate hundreds of organoids within a single focal plane, using reduced amounts of media and ECM reagents compared to standard culture methods.
This increased organoid quantity and decreased reagent usage enable researchers to enhance organoid generation scalability, ultimately improving downstream assays' throughput.
By leveraging image-based software-guided selection tools, organoids can be chosen based on size and other phenotypic characteristics. This enhances well-to-well reproducibility in subsequent assays and facilitates normalization across multiple arrays and experiments.
Lastly, the CellRaft AIR System offers an automated solution for isolating individual pre-characterized organoids into 96-well plates for downstream applications.
This, coupled with more streamlined workflows for organoid generation, fulfills the unmet requirement for automating the process of generating reproducible 96-well plates designed explicitly for organoid screening assays.
In summary, CellRaft Technology provides an efficient, cost-effective, and reproducible solution for advancing the use of these physiologically relevant organoid models in drug discovery and preclinical pipelines.
About Cell Microsystems
Making innovative tools that enable researchers to image, identify, and isolate viable single cells and clonal colonies
Researchers worldwide in the fields of CRISPR gene editing, oncology, stem cell biology, immunology and neurobiology use Cell Microsystems products, advancing sophisticated discovery across the life sciences.
The company’s CellRaft AIR® System addresses two widespread challenges facing scientists: the ability to actively select viable single cells or clonal colonies based on their phenotype, and match these cells to clonal expansion or molecular analyses. Cells are seeded, imaged, identified, and isolated on Cell Microsystem’s CellRaft® Arrays.
We have tested more than 100 Cell Lines with CellRaft Technology to demonstrate the high outgrowth efficiencies using our platform.
The company currently markets its products to researchers worldwide, and prides itself on being a customer-focused organization responsive to feedback and inspired to fuel deeper contributions to science.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.