Flow cytometers, droplet dispensers, and limiting dilution techniques aimed at depositing a single iPSC in a well, often create a strenuous and unnatural environment that leads to low clonal survival efficiency.
The CellRaft® Array provides a more biologically relevant culture environment by offering flask-like culture conditions combined with single-cell segregation, enabling tens of thousands of cells per consumable.
Given the unique capability of the CellRaft AIR® System to identify CellRafts containing specific numbers of cells at different time points, the researchers aimed to compare the efficiency of clonal formation when isolating cells at various stages along the exponential growth curve. The objective was to determine whether isolating a colony instead of a single cell could improve the success rates and efficiency of single-cell cloning workflows.
Benefits of the CellRaft AIR® System:
- Generate 100s of monoclonal iPSC lines in under two weeks
- Achieve >90% clonal outgrowth by isolating monoclonal colonies
- Enable all iPSC workflows from reprogramming to differentiation on one consumable
- Optimize iPSCs for 2D and 3D culture
Methods
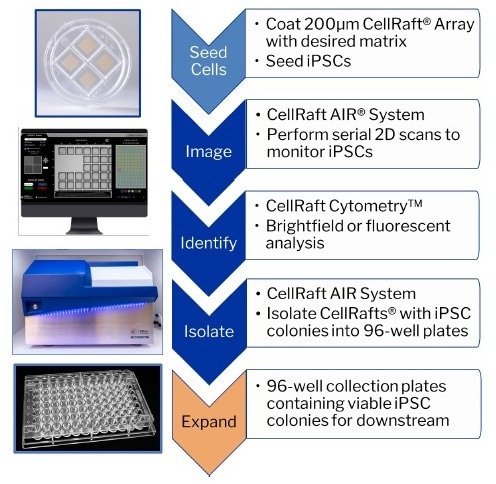
Image Credit: Cell Microsystems
Results
Only 10% of single iPSCs formed viable monoclonal colonies in the limiting dilution technique. Similarly, when using CellRaft technology at the single-cell stage of iPSC cell expansion, only 10% of the wells in the 96-well plates contained viable clones.
By isolating CellRafts at the four-cell and colony stages, the clonal outgrowth of iPSCs increased significantly to 87% and 98%, respectively.
Allowing the single cells to continue growing undisturbed in a shared microenvironment before colony isolation optimized the outgrowth efficiency, eliminating it as a limiting factor in cell line development workflows.
CellRaft technology supports various iPSC workflows, including the expansion of single cells, differentiation into different cell types or tissue-specific organoids, characterization of fluorescent expression or stained cells, and reprogramming human fibroblasts into iPSCs.
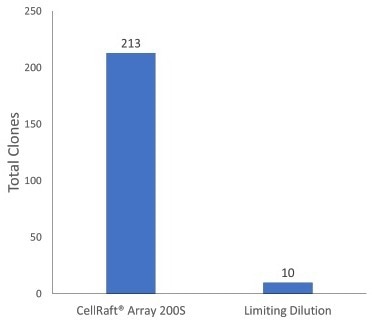
Image Credit: Cell Microsystems
Single human iPSCs were directly seeded onto an uncoated 200 µM Single CellRaft Array in dilute iMatrix-511 (Matrixome) at a density of 5:1 (cells: rafts). For example, limiting dilution plates were seeded at 1 cell/well thickness in five 96-well plates pre-coated with h-ESC Matrigel.
iPSCs on CellRaft® arrays
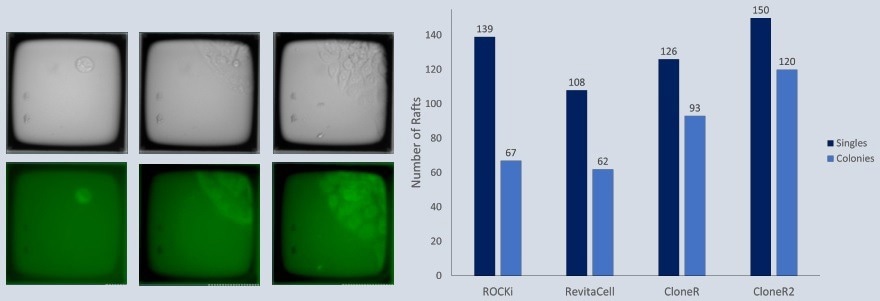
Image Credit: Cell Microsystems
Overnight in the incubator, a 200 m Single CellRaft Array was covered with iMatrix-511. GFP-labeled iPSCs were planted in mTesR Plus, dilute iMatrix-511, and different additives at a ratio of 5 cells per CellRaft. The array was serially scanned daily, and CellRafts with monoclonal colonies were separated utilizing the CellRaft AIR® System.
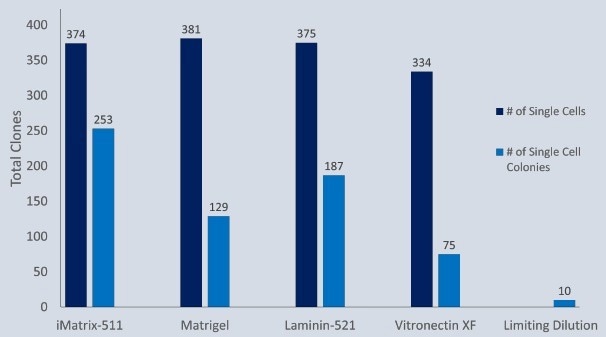
Image Credit: Cell Microsystems
In varied dilute concentrations of common matrices, iPSCs were seeded onto a 200 m Quad CellRaft Array. The CellRaft AIR System was used to identify and isolate single cell-derived iPSC colonies.
iPSC single cell expansion
Image Credit: Cell Microsystems
iPSCs were seeded in dilute iMatrix511 onto a 200 m Quad CellRaft Array. The array was sequentially scanned daily for three days to assess the effectiveness of clonal formation of iPSCs separated at various points along the exponential development curve to determine the best stage for dependable colony expansion.
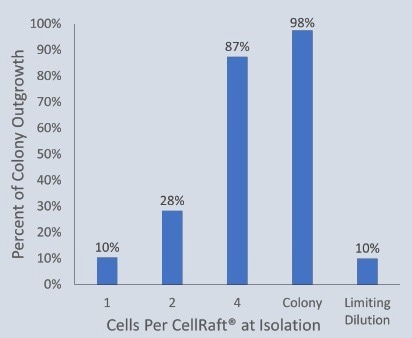
Image Credit: Cell Microsystems
The outgrowth efficiency of isolated CellRafts containing single iPSC-expanded cells was observed and compared with the limiting dilution plates.
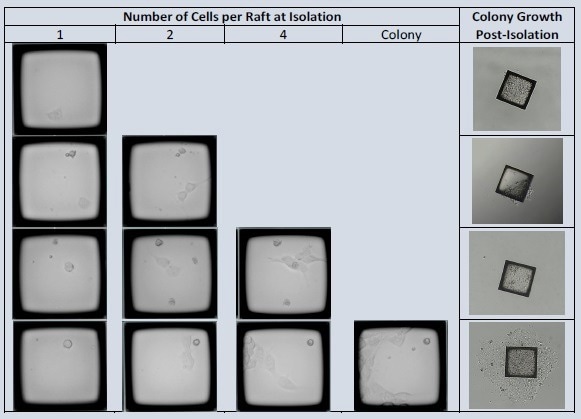
Image Credit: Cell Microsystems
The CellRaft Array was photographed four hours after seeding to detect CellRafts harboring iPSCs. CellRafts at various stages of development were isolated throughout the following three days. For one week after isolation, colony expansion was evaluated.
iPSC differentiated organoids
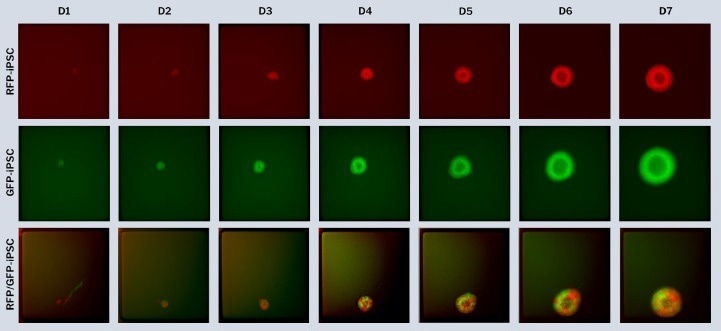
Image Credit: Cell Microsystems
Edited single iPSCs were differentiated to generate mono- and dual-fluorescent brain organoids on the 500 m CellRaft Array. RFP- and GFP-edited iPSCs were co-cultured in dilute ECM on the CellRaft Array for a week during embryoid body development and neural induction for choroid plexus organoid differentiation and photographed daily.
Reprogram iPSCs
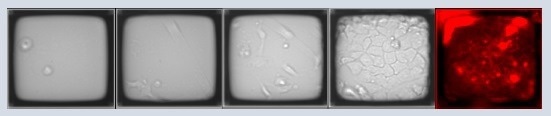
Image Credit: Cell Microsystems
A 200 µm Single CellRaft Array and a 6-well plate were coated with dilute Geltrex. Human fibroblasts were transfected and seeded onto the array or the 6-well plate. Daily imaging was conducted for both the array and the 6-well plate. After 15 days, cells were live stained for Tra-1-60 before isolation to confirm the pluripotency of reprogrammed iPSC clones.
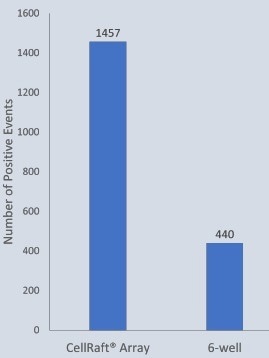
Image Credit: Cell Microsystems
About Cell Microsystems
Making innovative tools that enable researchers to image, identify, and isolate viable single cells and clonal colonies
Researchers worldwide in the fields of CRISPR gene editing, oncology, stem cell biology, immunology and neurobiology use Cell Microsystems products, advancing sophisticated discovery across the life sciences.
The company’s CellRaft AIR® System addresses two widespread challenges facing scientists: the ability to actively select viable single cells or clonal colonies based on their phenotype, and match these cells to clonal expansion or molecular analyses. Cells are seeded, imaged, identified, and isolated on Cell Microsystem’s CellRaft® Arrays.
We have tested more than 100 Cell Lines with CellRaft Technology to demonstrate the high outgrowth efficiencies using our platform.
The company currently markets its products to researchers worldwide, and prides itself on being a customer-focused organization responsive to feedback and inspired to fuel deeper contributions to science.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.