This article is based on a poster originally authored by Diana Chiluiza, Ph.D., Immune Cell Isolation Manager at BioIVT and Courtney Noah, Ph.D., Vice President of Scientific Affairs at BioIVT.
Human peripheral blood mononuclear cells (PBMCs) are key components of gene therapy and regenerative medicine research.1 PBMCs are made up of highly diverse immune cell populations, which contain lymphocytes such as CD4+ and CD8+ T cells, B cells, natural killer cells, monocytes, which can be grouped as macrophages and dendritic cells, and a small percentage of stem cells.2,3
Leukopaks (the product of leukapheresis collections) derived from a single donor are the favored source of PBMCs as they are rich in white blood cells and can reduce variability. Moreover, transport and storage conditions for leukopaks and other biospecimens have a significant impact on the quality of the product, measured not only as total cell numbers and viability but also as immune cell population diversity.
Using fresh leukopaks as starting materials accelerates key research and development processes, particularly when the leukopak collection and processing sites are in close proximity.
However, in circumstances that require long transit times, such conditions are not always possible.4 Local distribution of fresh leukopaks can take up to 12 hours, while deliveries to more remote areas can range from 24 to 48 hours, with international shipments taking even longer.
Previous studies have examined the effects of temperature and time on various parameters such as viability, morphology, phenotype retention, metabolism (including pH, glucose, O2 levels), mitochondrial DNA integrity, mitochondrial reactive oxygen species, membrane damage, and cytokine secretion in PBMCs and other cell lines.4-5
It was found that 24-hour ambient shipping had a minimal impact on PBMC activation by lipopolysaccharide and heat-killed Staphylococcus aureus, whereas a 48-hour duration led to complete abrogation of this activation.4 While shipping fresh products instead of frozen ones helps avoid cellular damage caused by osmotic pressure changes during cryopreservation, it comes with its own challenges.
Delivery delays can lead to temperature fluctuations in leukopaks, potentially affecting cell viability and altering metabolism and microtubule integrity. However, by carefully controlling shipping conditions and minimizing temperature changes, the undifferentiated phenotype of white blood cells can be preserved, which is essential for the development of novel therapies and the success of treatments.
Proper storage and shipping conditions for cell and gene therapy products are crucial for the design, repeatability, and reproducibility of research, development, product design, and therapy outcomes. To ensure this, the conditions of white blood cells in leukopak products were rigorously evaluated.
Over five days of storage at room temperature and at 4 °C to simulate shipping conditions, cell concentrations, viability, and differential leukocyte counts (DLC) were assessed.
Methods
Three healthy donors were randomly selected for leukapheresis. On the day of collection, at 0 hours, viability, CBC, and DLC were first assessed. From the remaining leukopak volume, 20 individual 1.5 mL aliquots were prepared and placed into 2 mL vials.
Ten vials were stored in room temperature shipping boxes (INTELSIUS PHT(15-25) 3.5L 60-hour temperature boxes), and the other ten were stored in cold shipping boxes (NanoCool™ Cooling System 2-8 °C 1.05 L shipping box), which were activated and sealed immediately.
ITAG Temperature Loggers were placed inside each box adjacent to the leukopak samples in order to monitor the temperature at 1-minute interval reads. Each day, CBC analysis, PBMC purification, and viability calculations using standard Trypan Blue Exclusion assays were performed after selecting two vials from each box.
Results
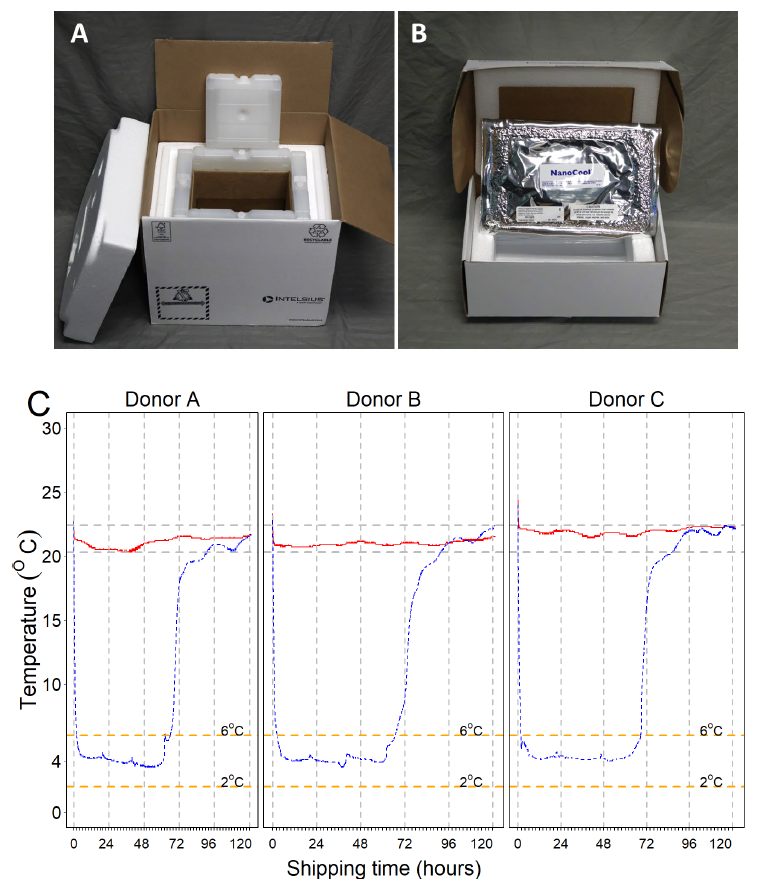
Figure 1. Ambient and cold boxes used in the study and temperature curves of the storage containers during the testing period. A. INTELSIUS PHT(15-25) 3.5 L 60-hr temperature box. B. NanoCool™ Cooling System 2-8 ºC 1.05 L shipping box. C. Temperature curves for room temperature and cold shipping boxes captured every minute for 5 days using ITAG temperature data loggers. Ambient boxes were able to maintain constant temperatures between 20.3 ºC and 22.4 ºC during the length of the study (red lines), with a mean of 21.3 ºC (SD 0.52). After activation of the NanoCool™ contents, the temperature inside our cold boxes (blue lines) dropped from room temperature to below 6 ºC in 2 hr, and it was maintained below 6 ºC for about 2.7 days (mean 67 hr), reaching room temperature on day 3. The mean temperature during the critical shipping time (below 6 ºC and above 2 ºC) was 4.2 ºC (SD 0.5). Image Credit: BioIVT
The results showed that during the first 24 hours of storage and transport under replicated conditions, cell viability remained relatively stable and comparable between the two temperature settings, with no significant differences in averages.
However, after 24 hours, viability began to decline rapidly in the room temperature group, reaching an average of 75.4 % (SD 11.6) by 48 hours. In contrast, viability in the cold storage group remained high, averaging 97.3 % (SD 2.3). The difference between these conditions was significant (p = 0.018).
By the 72-hour mark, viability remained stable in the cold conditions (average 96.6 %, SD 1.4) but dropped further in the room temperature group, with an average of 60.5 % (SD 13.2).
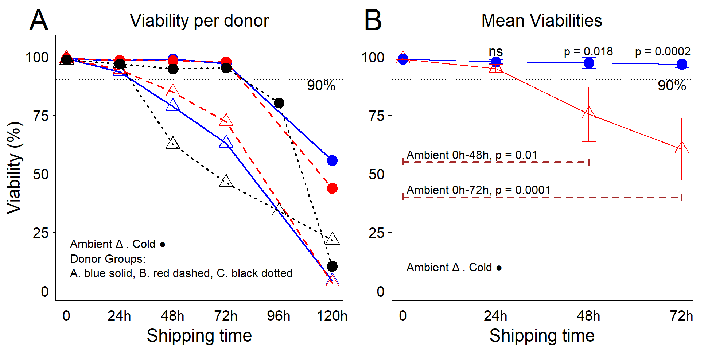
Figure 2. Temperature conditions influence the viability of freshly purified PBMCs during leukopak shipping. (A) PBMC viability per donor and per shipping time. (B) Mean viabilities per temperature and per shipping time. Error bars represent one standard deviation from the mean. Using robust cellular starting material for research and development is critical for the success advancement of novel therapies. The first measure for quality control (QC) is the percentage of viable cells in a product volume, and for industry standards, viabilities ≥90 % are preferred. Statistical analysis performed using ANOVA with post hoc Tukey’s Honest Significant Difference Test. Image Credit: BioIVT
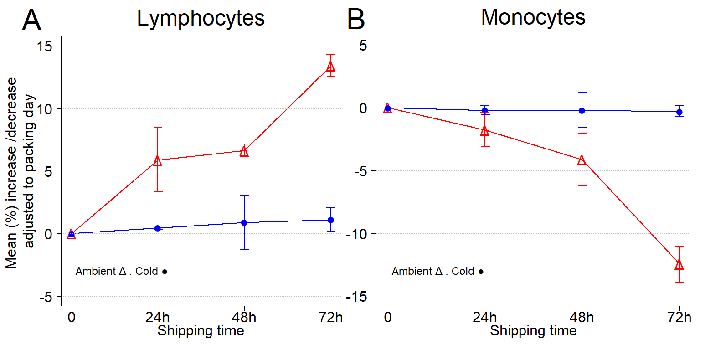
Figure 3. Temperature conditions (ambient versus 4 °C) influence the lymphocyte and monocyte composition of leukopaks during shipping. (A) Mean increase/decrease of lymphocytes per shipping day, adjusted to collection day. (B) Mean increase/decrease of monocytes per shipping day, adjusted to collection day. Error bars represent one standard deviation from the mean. Lymphocytes are the most important component of the immune system, including critical cell populations such as CD4+ and CD8+ T cells, NK cells, and antigen presenting CD19+ B cells. Monocytes, which differentiate into macrophages and dendritic cells, are another important component of the immune system involved in the phagocytosis of pathogens. CBC and DLC were obtained every day from three independent leukopak samples. Image Credit: BioIVT
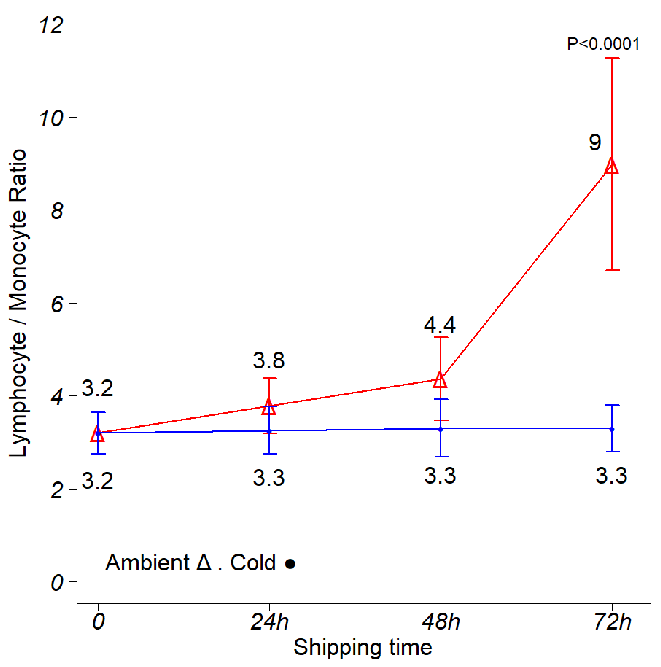
Figure 4. Effects of time and temperature on lymphocyte to monocyte ratios. Shipping conditions were mimicked using boxes stored at ambient temperature and 4 °C. Analysis was conducted using three independent leukopak samples. CBC and DLC were obtained every day, and the Lym/Mon ratio was calculated per donor, per shipping time and per storage temperature. The data points on the graph display the mean Lym/Mon ratio obtained during the study. The Lym/Mon ratio was stable during the first 72 hr in cold boxes, then it rapidly increases as the temperature in the cold boxes increases (Fig. 1C and data not shown). On ambient shipping, the Lym/Mon ratio shows a small but steady increase in the first 48 hr, rapidly jumping at 72 hr. Error bars represent one standard deviation from the mean. ANOVA with post hoc Tukey’s Honest Significant Difference Test. Image Credit: BioIVT
Conclusion
This study examines the impact of temperature variations during the shipping and storage of freshly collected leukopaks, comparing ambient (approximately 21 °C) and cold (4 °C) conditions.
Boxes stored at ambient conditions maintained a temperature of 21 °C for five days, while cold boxes, once activated, stabilized at 4 °C for approximately 72 hours before gradually returning to ambient temperature.
PBMC viability remained above 95 % for 72 hours under cold shipping conditions but dropped to 80 % by the 96-hour mark. In contrast, although ambient shipping showed a slight viability decline to 94 % at 24 hours, significant shifts were observed, including an increase in lymphocytes and a decrease in monocytes, particularly at 48 and 72 hours. Additionally, a rapid decline in viability was noted in ambient conditions, falling to 75 % at 48 hours.
Based on these findings, ambient shipping is recommended only for same-day local distribution, while cold shipping is strongly advised for longer distances or when potential transit delays could adversely affect cell health.
Further comparative studies exploring different shipping methods and assessing cell activation could provide additional valuable insights.
BioIVT aims to preserve cell product quality for up to 72 hours using NanoCool™ Cooling System shipping containers. Even with overnight shipping, maintaining cold conditions is highly recommended to ensure optimal cell health. By following these recommendations, researchers can avoid unexpected changes in leukopak cell populations and prevent potential testing failures.
References and further reading
- Kobayashi, D. T. et al. Evaluation of Peripheral Blood Mononuclear Cell Processing and Analysis for Survival Motor Neuron Protein. PLoS One 7, e50763 (2012).
- Abbas AK, L. A. Cellular and molecular immunology. vol. 5 (Saunders, Philadelphia, 2005).
- Jerram, A. et al. Effects of storage time and temperature on highly multiparametric flow analysis of peripheral blood samples; implications for clinical trial samples. Biosci Rep 41, (2021).
- Johnson, R. K., Overlee, B. L., Sagen, J. A. & Howe, C. L. Peripheral blood mononuclear cell phenotype and function are maintained after overnight shipping of whole blood. Sci Rep 12, (2022).
- Kim DW et al. Overnight storage of blood in ACD tubes at 4C increases NK cell fraction in peripheral blood mononuclear cells. Ann Clin Lab Sci 43, (2013).
About BioIVT
BioIVT, formerly BioreclamationIVT, is a leading global provider of high-quality biological specimens and value-added services. We specialize in control and disease state samples including human and animal tissues, cell products, blood and other biofluids. Our unmatched portfolio of clinical specimens directly supports precision medicine research and the effort to improve patient outcomes by coupling comprehensive clinical data with donor samples.
Our Research Services team works collaboratively with clients to provide in vitro hepatic modeling solutions. And as the world’s premier supplier of ADME-Tox model systems, including hepatocytes and subcellular fractions, BioIVT enables scientists to better understand the pharmacokinetics and drug metabolism of newly discovered compounds and the effects on disease processes. By combining our technical expertise, exceptional customer service, and unparalleled access to biological specimens, BioIVT serves the research community as a trusted partner in ELEVATING SCIENCE®.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.