Experiments utilizing cryopreserved primary hepatocytes (CPHs) can be effective and straightforward when best-practice handling procedures are followed. BioIVT provides standardized and optimized methodologies to help researchers produce the highest-quality hepatocytes in the industry.

Image Credit: BioIVT
Storage
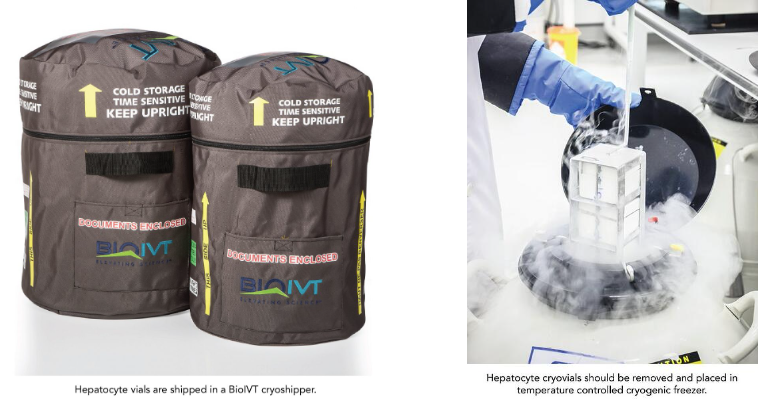
Image Credit: BioIVT
Proper storage of CPHs is vital to ensure that they maintain their features.
- Cells should be stored below -150 °C in liquid nitrogen vapor. Cells stored in liquid nitrogen may cause liquid to enter the vials, which can be hazardous when thawed.
- Cells should be transferred from the cryoshipper to a temperature-controlled freezer immediately after delivery.
- If necessary, vials can be transported under dry ice for a maximum of 30 minutes, but this method should be minimized.
Thawing
When thawing CPHs, the objective is to thaw them enough so that an ice pellet remains but is loose from the vial’s walls and can be transferred into a conical tube, preventing over-thawing and sustaining good cell viability.
BioIVT’s CPHs are unique in that they do not require purification or a wash stage to achieve high viability, saving researchers money and time.
- Before the handling of the cells, the appropriate INVITROGRO hepatocyte medium should be warmed to 37 °C.
- The cryoplateable hepatocytes can be thawed directly in a small volume of INVITROGRO CP medium (approximately 1 ml for every million cells per vial being thawed).
- Cells should be thawed immediately after removal from storage.
- When a small ice pellet remains after cells have pulled away from the vial wall, pour the contents into the pre-warmed INVITROGRO hepatocyte medium to complete the process.
Cell counting
Conducting accurate cell count is vital for ensuring that you have the correct cell concentration in the thawed cell suspension. Cell count errors can cause inaccuracies in incubation concentrations or cell density and affect results.
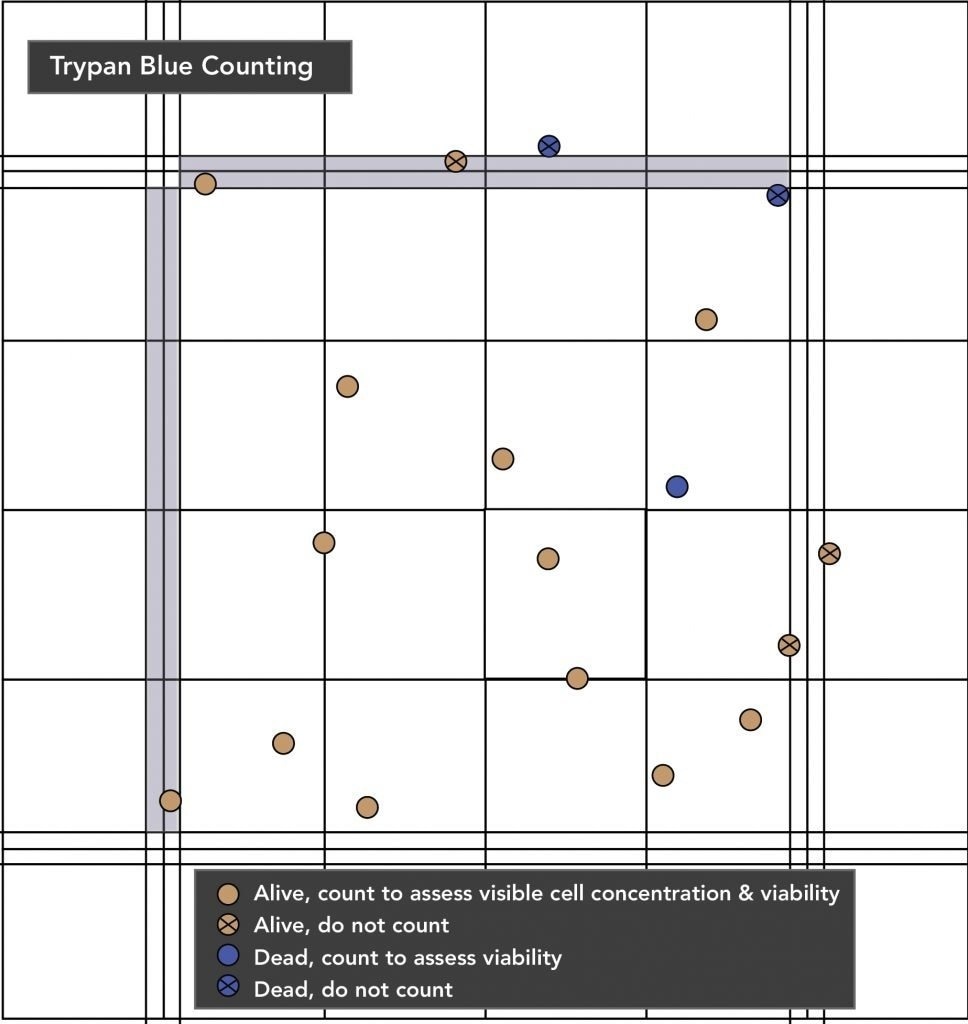
Image Credit: BioIVT
- The recommended method for determining cell concentration and viability is the Trypan Blue (TP) exclusion counting technique using a Neubauer hemocytometer.
- A 1:5 dilution of TP is recommended.
- The INVITROGRO KHB can be used to dilute Trypan Blue.
- (700 µL KHB + 200 µL TP + 100 µL cell suspension sample).
- To determine accurate cell viability, cells must be handled gently while in Trypan Blue.
- Cell counting must be performed within five minutes of cells being added to the Trypan Blue.
- Only two of the four sides of the square should be counted.
Hepatocytes have a high sedimentation rate, so cells should be resuspended before aliquoting or sampling (as shown in Figure 3).

Image Credit: BioIVT
Plating
Maintaining proper plating techniques allows researchers to seed and distribute the cells evenly to achieve maximum confluency.
Source: BioIVT
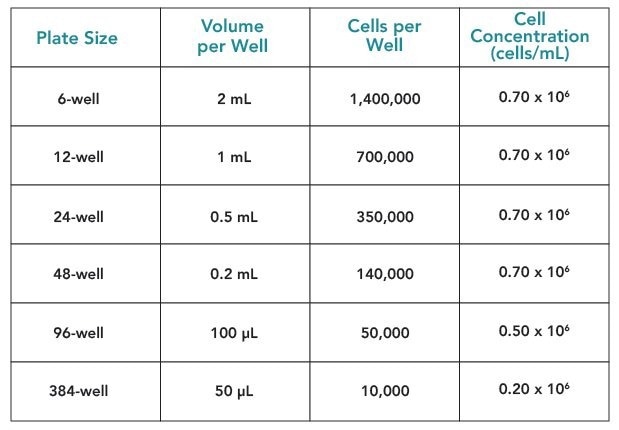
- Dilute the cells to 0.70 x 10 6 viable cells/mL with the recommended INVITROGRO hepatocyte medium for all species except mouse, or when utilizing 96 or 384-well plates.
- Mouse hepatocytes require half the seeding density as they are larger than those of other species. For instance, 0.35 x 10 6 viable cells/mL instead of 0.70 x 10 6 viable cells/mL.
- To avoid over- or under-seeding, seed at the correct concentrations.
- To distribute the cells evenly, shake the plate gently in a north-south and then east-west direction.
- To remove any cells that did not attach, change the medium three to four hours after plating.
Product selection
Researchers need to select the correct product for their research.
- Know whether an assay requires cryoplateable or suspension hepatocytes.
- BioIVT’s team can support you, helping you choose the optimal products for your application.
- BioIVT has LIVERPOOL® Cryoplateable and Cryosuspension Human Hepatocyte lots of up to 200 donors to meet the diverse pooled donor needs of its customers.
- BioIVT’s comprehensive human donor liver inventory enables the screening of specific characteristics, such as genotypes, HLA, pathology, demographics, CYP450 fold induction values and transporter function.
About BioIVT
BioIVT, formerly BioreclamationIVT, is a leading global provider of high-quality biological specimens and value-added services. We specialize in control and disease state samples including human and animal tissues, cell products, blood, and other biofluids. Our unmatched portfolio of clinical specimens directly supports precision medicine research and the effort to improve patient outcomes by coupling comprehensive clinical data with donor samples.
Our Research Services team works collaboratively with clients to provide in vitro hepatic modeling solutions. And as the world’s premier supplier of ADME-Tox model systems, including hepatocytes and subcellular fractions, BioIVT enables scientists to better understand the pharmacokinetics and drug metabolism of newly discovered compounds and the effects on disease processes. By combining our technical expertise, exceptional customer service, and unparalleled access to biological specimens, BioIVT serves the research community as a trusted partner in ELEVATING SCIENCE®.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.