BioIVT views its clients as partners and works to design and implement absorption, distribution, metabolism, and excretion (ADME) and drug-drug interaction (DDI) programs that align with their specific research goals, meet decision-making needs, and accelerate R&D timelines.

Image Credit: BioIVT
The company's value-driven, streamlined laboratory processes support reliable and informative scientific study designs, ensuring high-quality results.
Its laboratory in Kansas City, KS, boasts a custom-designed data management infrastructure that features an advanced and comprehensive electronic laboratory notebook (ELN) system.
By incorporating a state-of-the-art ELN into its already efficient data management system, BioIVT was able to lower costs and study timelines for a range of its clients.
These efficiencies facilitated the development of standard-tiered in vitro ADME/DDI study options. Design and deliverable options can be tailored to each stage of the preclinical drug development process, while each client’s preferred in/outsourcing strategy and risk management approach can be accommodated.
Digital transformation
BioIVT launched its ELN and data management system following extensive design, configuration, and validation work. The company continues to refine and improve its processes, with its digital transformation journey centered around the comprehensive evaluation and refurbishment of its lab operation processes.
This approach is key to ensuring streamlined, efficient workflows while continuing to adhere to the 21 CFR Part 58 (Good Laboratory Practice, GLP) and Part 11 (Electronic Records and Electronic Signatures) standards.
The revamp introduced barcode-based chemical and equipment management, ensuring seamless documentation of study data and foundational facility records. It also integrated business rule data and QC checks into workflows, significantly reducing turnaround times for clients.
The system led to a dramatic reduction in paper records, enabled software-assisted data review, and minimized the need for manual QC checks. This helped Study Directors deliver data to clients more quickly while maintaining BioIVT's rigorous quality standards.
Taking a tiered approach
BioIVT’s clients operate according to strict research and development timelines. Accurate study data is, therefore, critical to decision-making, and every day counts when working towards a research goal.
The company aimed to develop an efficient end-to-end solution that was focused on value-rich endpoints. The system was required to reduce a busy workflow to quickly deliver client-ready data, while supporting assay documentation, processing all relevant data, and maintaining rigorous scientific review.
BioIVT offers a series of fit-for-purpose study designs:
- Tier 1 (Basic Program): Developed to enable decision-making and inform future definitive studies.
- Tier 2 (IND-Enabling Program): Developed to provide IND submission-ready data and comprehensive standard reports.
- Tier 3 (IND-Enabling Program with eCTD Report): Developed to offer the detail and rigor of Tier 2, with the addition of eCTD readiness and the option of GLP compliance.
Understanding BioIVT’s study tiers
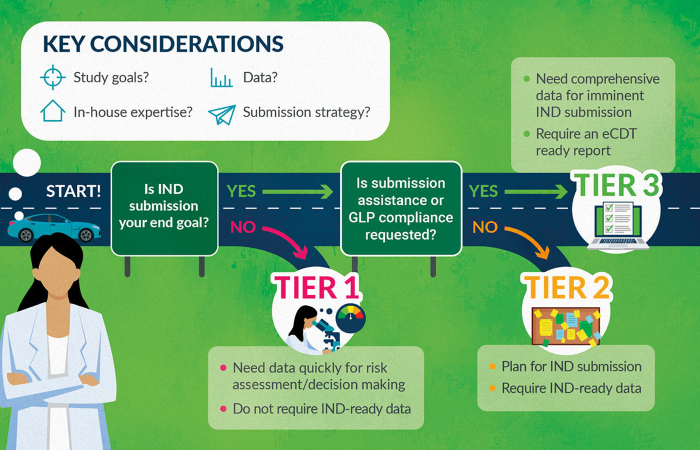
Image Credit: BioIVT
Each tier offers progressively faster timelines. Tier 1 is designed to address specific questions and provide early ADME property and DDI risk assessments, which inform definitive study design, drug development strategies, and decision-making.
While Tier 1 and Tier 2 studies have different designs, both deliver the same comprehensive report. Tier 2 and Tier 3 provide the necessary data and reports to support an Investigational New Drug (IND) application to the FDA. Tier 3 also includes Electronic Common Technical Document (eCTD) formatting, which is compliant with FDA, EMA, and ICH electronic submission standards.
In research settings where none of BioIVT’s standard designs can accommodate a client’s needs, the company will work alongside the client to customize a study to meet their specific research goals.
This innovative three-tiered approach was launched for nine study types:
- CYP induction
- CYP inhibition
- CYP reaction phenotyping
- UGT inhibition
- UGT reaction phenotyping
- Metabolic stability
- Metabolite ID
- Transporter substrate
- Transporter inhibition.
All of these studies provide the level of data integrity, data quality, and scientific expertise expected from a laboratory with over 25 years experience in this field, but with a much more rapid turnaround time than would normally be available.
For example, BioIVT has been able to reduce the study cycle time for its Tier 2 CYP Inhibition and CYP Induction studies by approximately 1 month. The resulting efficiencies and labor savings have also led to reduced pricing for customers.
CYP induction scenarios
The following scenarios presented here successfully demonstrate the capabilities of this new tiered system.
Scenario A
The client is planning an IND submission for next year and needs to design an enzyme induction study. Their compound class tends to up-regulate the pregnane X receptor (PXR), and they are considering whether to include CYP2Cs. Being budget-sensitive, they want to ensure the study design is correct from the outset.
BioIVT would recommend a Tier 1 induction study using a single, sensitive donor for CYP1A2, 2B6, and 3A4. The mRNA data can help inform the full study design. If up-regulation is observed, it would be beneficial to include the CYP2Cs from the beginning, potentially saving weeks compared to a stepwise approach.
Scenario B
In this scenario, the same client is now ready for their IND-submission study. The Tier 1 study confirmed PXR up-regulation. They remain time- and budget-conscious but require the standard EC50/Emax endpoints.
Although the client has internal and external support for assembling the submission package, they may still choose to follow an exit strategy prior to the IND submission. In this case, BioIVT would recommend a Tier 2 CYP induction study, including options for CYP2Cs with activity.
Scenario C
About six months after finalizing the study, the same client changes their strategy due to a company acquisition. They now want to submit the report directly to the FDA’s e-submission system. The client has several options:
- They can request an upgrade to a Tier 2 report suitable for e-submission prepared by one of BioIVT’s technical writing experts. This is the fastest and most cost-effective option.
- If they prefer a more detailed discussion, they can opt for a Tier 3 report, which has a different format. Since the study designs and data for Tier 2 and Tier 3 are identical, this option is slightly slower and mid-range in cost.
- The final option is a custom report tailored to meet their internal technical formatting requirements, which is the slowest and most expensive approach.
Scenario D
A client requires a CYP induction study for an IND submission, and their regulatory department or another stakeholder has requested that everything, including the in vitro DDI studies, be conducted under GLP standards. In this case, the Tier 3 GLP CYP induction study is the best option.
Scenario E
A client requires an IND-friendly CYP induction study but has non-standard design requirements. For instance, they request positive control inducers at all seven concentrations and need EC50/Emax data, which is uncommon. Additionally, the client has specific internal report format and content requirements.
In this case, BioIVT would engage its technical advisory team to develop a custom induction study tailored to the client’s needs.
Conclusion
BioIVT has leveraged its extensive digitization and automation expertise and achievements to develop tailored, high-quality, tiered ADME services on behalf of its clients. This approach allows the company to continue to offer scientifically rigorous studies at a lower cost and faster while meeting each individual client’s ADME research requirements.
Acknowledgments
Produced from materials originally authored by Dr. Joanna Barbara from BioIVT.
About BioIVT
BioIVT, formerly BioreclamationIVT, is a leading global provider of high-quality biological specimens and value-added services. We specialize in control and disease state samples including human and animal tissues, cell products, blood and other biofluids. Our unmatched portfolio of clinical specimens directly supports precision medicine research and the effort to improve patient outcomes by coupling comprehensive clinical data with donor samples.
Our Research Services team works collaboratively with clients to provide in vitro hepatic modeling solutions. And as the world’s premier supplier of ADME-Tox model systems, including hepatocytes and subcellular fractions, BioIVT enables scientists to better understand the pharmacokinetics and drug metabolism of newly discovered compounds and the effects on disease processes. By combining our technical expertise, exceptional customer service, and unparalleled access to biological specimens, BioIVT serves the research community as a trusted partner in ELEVATING SCIENCE®.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.