The field of cell and gene therapy has experienced remarkable growth over the last ten years. Autologous and allogeneic therapies have extended their reach across various indications, such as oncology, regenerative medicine, and genetic disorders.
Traditional approaches to developing manufacturing processes for these therapies, as well as the testing of consumables, media and reagents, and ancillary equipment, involve starting material derived from healthy donors.
However, do these “normal” cells accurately represent the real world, where starting materials are sourced from patients with diseases?
To address this concern, researchers should consider implementing verification and validation (V&V) testing utilizing disease-state leukopaks and disease-state immune cell subsets to guarantee that they are well-suited for autologous applications.
Defining “disease state”
An individual who has been clinically diagnosed with a specific disease state is a disease state donor. However, disease progression, previous treatments, and co-morbidities can significantly affect the resultant severity of the disease at the time of sample collection.
Suppliers of disease state material must therefore document the patient’s full clinical history. BioIVT’s clinical collection sites offer a comprehensive clinical data set for its products, complete with over 300 relevant data points. This enables customers to develop their own court of disease-state patients for their specific V&V strategies.
The ethical collection of products from donors is equally as important as the quality of patient material and clinical data. Patients debilitated by disease should not be burdened with additional procedures that could impair their outcome.
BioIVT takes pride in its IRB-approved, donor-consented collection techniques that prioritize patient safety. As a result, the majority of leukopak collections are restricted to a half-pak size for disease-state donors.
Other groups, such as oncology disease states, are limited to even smaller volumes to decrease the effect on donor health and safety. Immune cell subsets obtained from disease-state donors are isolated according to qualified standard operating procedures (SOPs) to guarantee that no product is wasted.
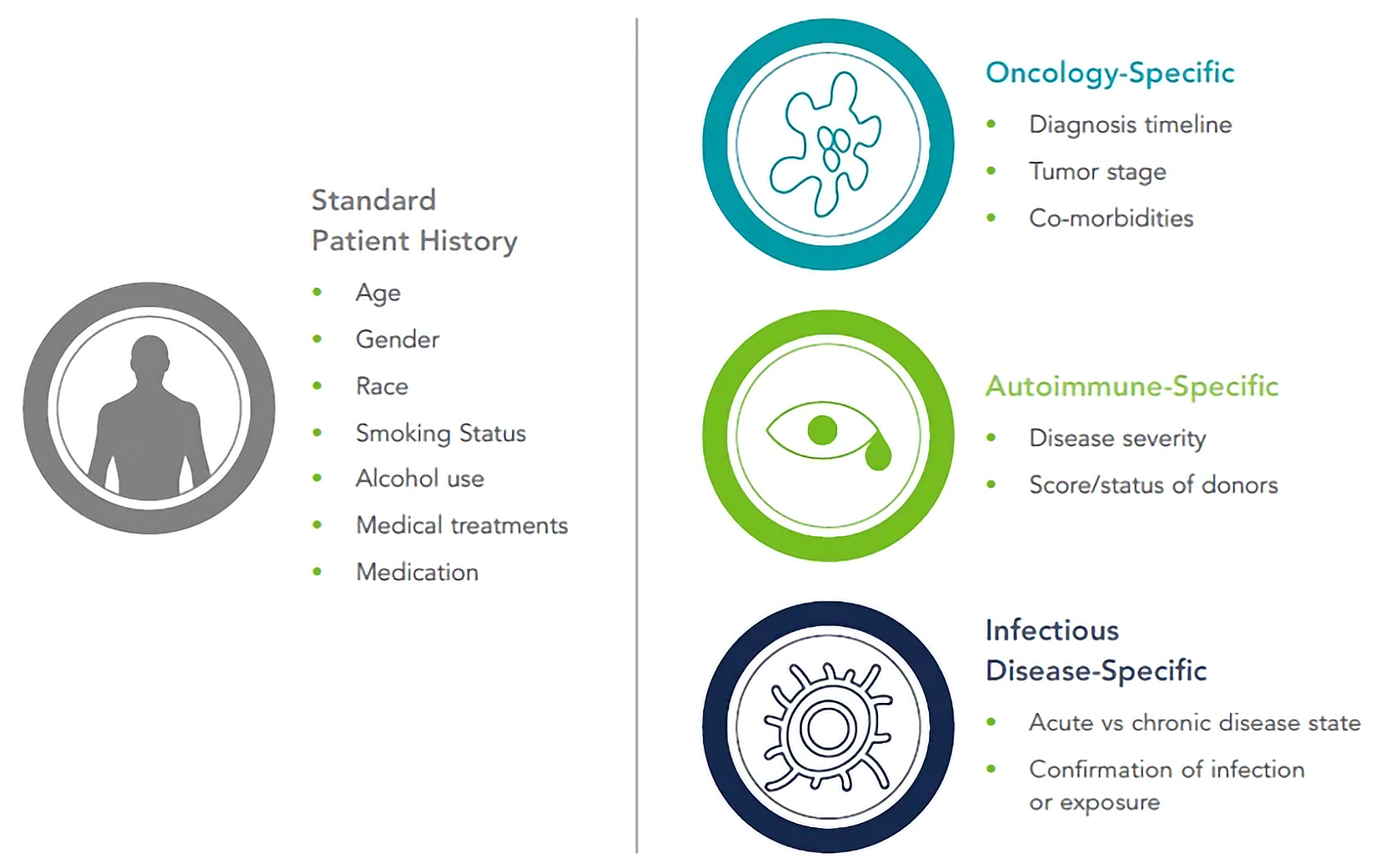
Image Credit: BioIVT
Differences between normal donors and disease-state donors
While the achievements of autologous cell therapies in the hematology/oncology field have been substantial, they are not yet deemed as a first-line treatment.
According to MD Anderson Cancer Center, only patients who have “been through two lines of unsuccessful treatment can get the FDA-approved commercial product.”1 Each FDA-approved therapy has an extensive set of prior approval standards before patients can obtain the medication.2-5
Source: BioIVT
| Therapy Name |
Indications |
Manufacturer |
Prior Authorization Criteria |
| KYMRIAH® (tisagenlecleucel) |
|
Novartis |
- 3-25 years of age
- Refractory or relapsed disease
- Failed 2+ cycles of chemotherapy
- Documentation of CD19 tumor expression
- No active infections
|
| YESCARTA® (Axicabtagen Ciloleucel) |
- DLBCL
- Non-Hodgkin lymphoma
|
Kite Pharma |
- 18 years of age or older
- Refractory or relapsed disease
- Failed anthracycline-containing chemotherapy regimen
- CD20 expression (for CD20+ disease)
- No active infections
|
| TECARTUS™ (Brexucabtagene autoleucel) |
|
Kite Pharma |
- 18 years of age or older
- No active infections
- No prior allogeneic hematopoietic stem cell transplantation
- No central nervous system lymphoma
- Relapsed or refractory disease
- At least one previous systemic therapy failure
|
| ABECMA™ (idecabtagene vicleucel) |
|
Brisol Myers-Squibb |
- 18 years of age or older
- At least 4 prior treatment regimens including 1 IMiD® agent, proteasome inhibitor, or anti-CD38 antibody
- Relapsed or refractory disease
|
As a result of these earlier treatments, typically chemotherapy and radiotherapy, the quality of the patient’s cells is reduced. The decreased production of cells, diminished production of cytokines, and fluctuations in the populations of immune cell subsets have been reported in the presence of common anticancer agents.6-8
Even conventional CD3/CD28 activation techniques executed on chemotherapy-exposed T cells can lead to diminished levels of proliferation relative to untreated cells.9
These activation techniques can also cause low transduction efficiency—that is, the number of T cells that are converted to chimeric antigen receptor (CAR) T cells by viral vector.10
These small changes represent a set of irregularities that are not captured in conventional V&V testing utilizing only healthy-patient material.
While oncology remains the main focus for much of the cell and gene therapy industry, ex vivo cell therapies exist for other indications, like autoimmune disorders.
Repairing deficiencies and defects of immunosuppressive regulatory T cells in “tolerizing cellular therapies” offers an innovative solution for conditions like systemic lupus erythematosus (SLE), Crohn’s disease, and graft versus host disease (GVHD).11
Regenerative medicine treatments derived from autologous mesenchymal stem cells (MSCs) or induced pluripotent stem cells (iPSCs), like those for Type 1 diabetes,12 may also benefit from starting materials derived from relevant diseases.
As these fields further progress, researchers may seek to validate the applicability of their results to real-world scenarios by comparing their manufacturing methods to true disease-state patient material.
Disease-state products for verification and validation of devices and reagents
The advantages of disease-state products extend beyond therapeutic research. Manufacturers of bioreactors, cell culture vessels, and other cell processing kits or devices often validate their products using healthy donor cells to demonstrate reproducibility and reliability.
While crucial for the launch of any cell and gene therapy, incorporating representative data sets from appropriate disease state material would help manage expectations for the end-users and provide insights into process optimization.
Cell culture media and reagent suppliers can also refine formulations to better suit real-world applications, saving their customers time and enhancing patient outcomes.
BioIVT’s portfolio of disease state leukopaks and immune cell subsets is strategically designed to offer researchers increased data accuracy that cannot be found with healthy starting material alone.
References and further reading
- Demarco, Cynthia. (2018) 9 things to know about CAR T-cell therapy. MD Anderson Cancer Center
- Prior Authorization Criteria: Kymriah (tisagenlecleucel). FirstCare. https://www.firstcare.com/en/Home
- Prior Authorization Criteria: Yescarta (axicabtagene ciloleucel). FirstCare. https://www.firstcare.com/en/Home
- Prior Authorization Criteria: Tecartus™ (brexucabtagene autoleucel) (Intravenous). Medica. https://specialtydrug.magellanprovider.com/media/235455/tecartus.pdf
- Abecma (idecabtagene vicleucel) Package Insert. FDA. https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagenevicleucel
- Sakai, H. et al. (2013). Effects of anticancer agents on cell viability, proliferative activity and cytokine production of peripheral blood mononuclear cells. J Clin Biochem Nutri., 52(1): 64-71
- Larsson, A., A. Roxa, K. Leandersson & C. Bergenfelz. (2019). Impact of systemic therapy on circulating leukocyte populations in patients with metastatic breast cancer. Scientific Reports, 9, 13451
- McDonnell, A. et al. (2017). Serial immunomonitoring of cancer patients receiving combined antagonistic anti-CD40 and chemotherapy reveals consistent and cyclical modulation of T cell and dendritic cell parameters. BMC Cancer, 17, 417
- Das, R., R. O’Connor, S. Grupp & D. Barrett. (2020). Lingering effects of chemotherapy on mature T cells impair proliferation. Blood Adv., 4(19): 4653-4664
- Pampusch, M. et al. (2019). Rapid Transduction and Expansion of Transduced T Cells with Maintenance of Central Memory Populations. Molecular Therapy Methods & Clinical, 16, 1-10
- Mosanya, C. & J. Issacs. (2019). Tolerising cellular therapies: what is their promise for autoimmune disease? Annals of the Rheumatic Diseases, 78: 297-310
- Matveyenko, A. & A. Vella. (2015). Regenerative Medicine in Diabetes. Mayo Clin Proc., 90(4): 546-554
About BioIVT
BioIVT, formerly BioreclamationIVT, is a leading global provider of high-quality biological specimens and value-added services. We specialize in control and disease state samples including human and animal tissues, cell products, blood, and other biofluids. Our unmatched portfolio of clinical specimens directly supports precision medicine research and the effort to improve patient outcomes by coupling comprehensive clinical data with donor samples.
Our Research Services team works collaboratively with clients to provide in vitro hepatic modeling solutions. And as the world’s premier supplier of ADME-Tox model systems, including hepatocytes and subcellular fractions, BioIVT enables scientists to better understand the pharmacokinetics and drug metabolism of newly discovered compounds and the effects on disease processes. By combining our technical expertise, exceptional customer service, and unparalleled access to biological specimens, BioIVT serves the research community as a trusted partner in ELEVATING SCIENCE®.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.