CYP suppression can be understood as a downregulation of gene expression, more specifically, a reduction in the gene transcription rate. Quantitative PCR is generally employed in the measurement of this process, which decreases the enzymatic activity of selected drug-metabolizing enzymes.
Understanding immunomodulators
In the broadest sense, immunomodulators are drugs that adjust immune responses, for example, modulation of cytokine levels.
Cytokines are signaling proteins responsible for the regulation of immune responses and other cellular processes, including the transcription of drug-metabolizing enzymes and transporters. Pro-inflammatory cytokines are known to suppress cytochrome P450 (CYP) enzymes.
Key human proinflammatory cytokines include:
Source: BioIVT
| |
|
| interleukin-1, IL-1 |
tumor necrosis factor alpha, TNF- α |
| interleukin-6, IL-6 |
interferon gamma, IFN-γ |
| interleukin-12, IL-12 |
granulocyte-macrophage colony-stimulating factor, GM-CSF |
| interleukin-18, IL-18 |
|
It is important to note that BioIVT uses l-2 and Il-6 as positive controls for the responsiveness of the whole human.
Immunomodulators are large molecules such as:
- Antibodies such as TGN-1412N (Engl J Med 2006), and muromonab (Clin Transplant. 1997).
- Recombinant cytokines such as rhIl-2 (Pharmacotherapy 1994) and rhIl-18 (Clin Cancer Res. 2006)
- Cytokine receptors such as soluble Il-6 receptor (Clin Cancer Res. 2006)
- Vaccines and vaccine adjuvants such as (Clin Cancer Res. 2006)
Small immunomodulator molecules include:
- Tofacitinib and JAK inhibitors are frequently employed in rheumatoid arthritis management.
- Disease-modifying drugs used to treat multiple sclerosis, for example, cladribine, cyclophosphamide, azathioprine, methotrexate, and mitoxantrone
- Thalidomide analogs used to treat multiple myeloma
BioIVT typically focuses its work on immunomodulators that are able to affect proinflammatory cytokine expression.
Immunomodulator-mediated drug interactions
A range of external factors (1) can initiate a cascade of cellular events culminating in CYP suppression. This can result in a number of physiological responses, including fever, higher levels of proinflammatory cytokines, and changes in acute-phase reaction proteins (2).
In terms of drug safety, the suppression of drug-metabolizing enzymes and transporters can lead to increased exposure to hepatically cleared drugs, potentially resulting in exaggerated pharmacological responses and/or drug toxicity (3).
Source: BioIVT
| |
|
| 1 |
- Viral and bacterial infection, LPS • Vaccination
- Inflammatory or chronic disease, cancer, trauma
- Certain therapeutic proteins, immunomodulator drugs
|
| 2 |
- Fever
- Increased proinflammatory cytokines IL-1, IL-6, TNF alpha, INF gamma
- Acute phase reaction proteins (C-reactive protein, complement factors, alpha1 acid glycoprotein)
- Changes in the activity of nuclear receptors, e.g., CAR, PXR, HNF, NF-kB
|
| 3 |
- Suppression of drug-metabolizing enzymes and transporters
- Increased exposure to hepatically cleared drugs
- Drug toxicity and/or exaggerated pharmacology
|
Cytokine-mediated drug interactions are exemplified by several notable cases:
- The interaction between the monoclonal antibody Muromonab-CD3 (Orthoclone OKT3) and cyclosporine (CSA) in renal transplant recipients, where OKT3 therapy caused a significant rise in CSA trough levels (Clin Transplant, 1997).
- The interaction between the IL-2 receptor antibody basiliximab and CSA levels in pediatric liver transplant recipients, where antibody-mediated suppression of CYP3A4 necessitated a reduction in cyclosporine dosage (Lancet, 2000).
- The clinically significant drug interaction between basiliximab (BASI) and tacrolimus (TAC) in renal transplant recipients, where BASI-treated patients experienced increased TAC blood trough levels on day 3 compared to controls (Transplant Proc., 2002).
- Tocilizumab's reversal of IL-6-induced suppression of CYP3A4 activity in rheumatoid arthritis (RA) patients, where exposure to simvastatin, a CYP3A4 substrate, was significantly reduced at 1 and 5 weeks after tocilizumab infusion in 12 RA patients (Clin Pharmacol Ther., 2011).
- The infamous case of the TGN1412 super-agonistic monoclonal antibody (mAb), a potent cytokine release agent, which led to a cytokine storm during a first-in-human clinical trial (N Engl J Med, 2006).
Regulatory guidance: ICH drug interaction studies M12 guideline
The 2024 global ICH guidance highlights the role of proinflammatory cytokines in regulating CYP expression.
Proinflammatory cytokines and immunomodulating drugs that stimulate cytokine release can suppress drug metabolism. Conversely, therapeutic proteins that lower elevated cytokine levels, such as tumor necrosis factor inhibitors, can reverse CYP down-regulation caused by an inflammatory environment (e.g., in rheumatoid arthritis). This process, referred to as de-suppression, increases CYP expression and activity, thereby reducing exposure to CYP substrates (ICH M12 guidance).
An example of de-suppression can be seen in rheumatoid arthritis, where suppression of CYP activity may be alleviated by immunosuppressive drugs like the anti-IL-6 receptor antibody tocilizumab.
A 2012 FDA guidance noted that “In vitro or animal studies have limited value in the projection of clinical interactions” (FDA 2012), reflecting the limitations of these studies in accounting for indirect, cytokine-mediated effects of immunomodulators on drug metabolism and transport.
The ICH guidance also points out that antibody-drug conjugate (ADC) payloads can act as immunomodulators, such as in the case of antibody-siRNA conjugates (Int J Pharm., 2021). Additionally, monoclonal antibodies (mAbs) themselves may target receptors involved in the immune response.
BioIVT study design
BioIVT employs a stepwise approach for the evaluation of new chemical entities’ immunomodulating potential:
- Is the test article an immunomodulator?
- Does the plurality of stimulated cytokines result in CYP suppression?
The company’s innovative study design distinguishes between the direct (left-hand side of the flow chart) and cytokine-mediated effects of test articles (right-hand side of the flow chart).
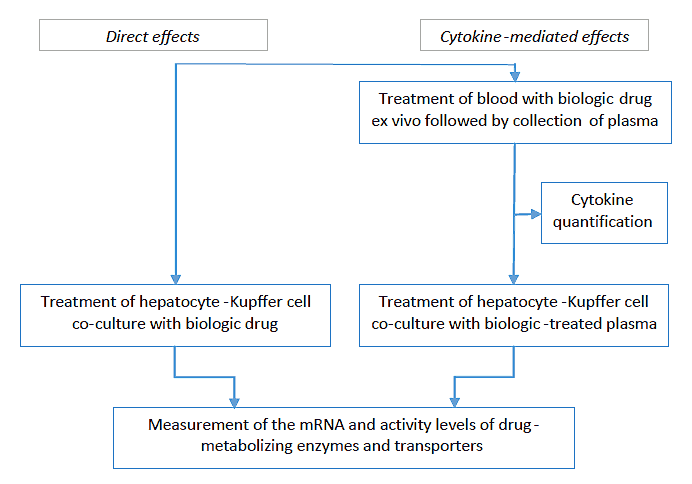
Image Credit: BioIVT
A cytokine being developed as a therapeutic agent can directly suppress CYP enzymes. An immunomodulator, such as an anti-CD28 monoclonal antibody (mAb), can stimulate the release of cytokines from peripheral blood mononuclear cells (PBMCs), which in turn may influence CYP expression in hepatocytes.
Conclusions
As the number of drugs interacting with the human immune system grows, so does the need to identify immunomodulators that have the potential for pharmacokinetic interactions with co-administered medications.
In vitro studies performed by BioIVT have enabled the identification a range of drugs prompting the release of proinflammatory cytokines. These drugs have been widely recognized for their ability to suppress drug-metabolizing enzymes and transporters.
The first step in BioIVT’s approach involves identifying a drug as a potential hazard for cytokine release. The second step quantifies the combined effects and variety of stimulated cytokines in human hepatocytes. This data is then used to guide further development of the compound.
Acknowledgments
Produced from materials originally authored by Maciej Czerwinski, Ph.D. from BioIVT.
About BioIVT
BioIVT, formerly BioreclamationIVT, is a leading global provider of high-quality biological specimens and value-added services. We specialize in control and disease state samples including human and animal tissues, cell products, blood and other biofluids. Our unmatched portfolio of clinical specimens directly supports precision medicine research and the effort to improve patient outcomes by coupling comprehensive clinical data with donor samples.
Our Research Services team works collaboratively with clients to provide in vitro hepatic modeling solutions. And as the world’s premier supplier of ADME-Tox model systems, including hepatocytes and subcellular fractions, BioIVT enables scientists to better understand the pharmacokinetics and drug metabolism of newly discovered compounds and the effects on disease processes. By combining our technical expertise, exceptional customer service, and unparalleled access to biological specimens, BioIVT serves the research community as a trusted partner in ELEVATING SCIENCE®.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.