Cerba Research, previously Barc Lab, is extremely committed to delivering comprehensive central lab services.

Image Credit: Cerba Research
When working in partnership for global central laboratory services, Cerba Research’s industry-leading laboratories spanning five continents, and 35 years of experience, allow the company to focus on trial success.
In 2016, Cerba Research collaborated with mid-level biotech for the largest phase III nonalcoholic steatohepatitis (NASH) study to date.
NASH touches almost 12% of people in developed countries, with an increasing number of patients advancing to end-stage liver disease. There is an urgent need for NASH therapeutic agents, so Cerba Research is keen to help.
Central lab services provided by Cerba for this study included:
- Biomarker testing
- Global liver biopsies, slide handling, and processing for KOL investigator
- Project management
- Safety analysis, patient monitoring for response to drug and placebo
- Site support
- Unified global study database
In NASH screening, the liver biopsy is the gold standard
Managing global clinical trial testing can be quite a challenge, and NASH is indicative of an operation with real complexity.
To be successful, Cerba needed to leverage all of their experience — and a significant number of local affiliates that were NASH-knowledgeable — to manage the sheer number of sites, countries and patients involved.
Between 2016 and 2019, the work included:
31 countries
>600 sites
>2000 patients randomized
>5000 liver biopsies
NA, EU, AUS, AF
NASH study complexity extends beyond liver biopsy collection
Liver biopsy results tend to be the principal endpoint in monitoring the progress of NASH disease while remaining the gold standard for diagnosis. During NASH clinical trials, biopsy processing, staining and reporting are critical steps.
Through experience, flexibility and attention to detail, Cerba was able to implement powerful quality processes at each step of the complex biopsy workflow for more than 5000 patients screened at over 600 sites in 31 countries.
The Cerba teams applied expertise in:
- Custom staining
- Digital imaging and software analysis of stained samples
- Flexibility to adjust processes toward agency updates/requirements
- In-house NASH testing
- Liver biopsy performance
- Logistics around biopsy blocks and slides
- Relationship building with key opinion leaders (KOL)
Reading the slight histological changes in NASH biopsies is effectively an art form mastered by a small number of KOLs around the world.
Outstanding cooperation with the top pathologists combined with expert slide preparation — including digital imaging and analysis — made sure the biopsy readings delivered were consistent throughout this successful study.
The right in-house validated biomarker testing makes all the difference
From noninvasive tests for fibrosis scoring to biochemical urine markers to liver tissue tests, NASH trials can demand a large range of validated testing, from simple to exploratory options. With a trial like this one spanning the entire globe, study teams depend on easy data access and comparability of results.
As Cerba Research provides guidance and carries a large portfolio of biomarkers necessary for NASH and other trials, the client faced no obstacles when securing what was needed. Even assays that Cerba does not usually carry can be added and validated in-house.
Typically, the biomarker testing covers:
- Genetic predisposition
- Glycemic and lipid biomarkers
- Inflammatory biomarkers
- Safety and serology
Lab support for NASH studies has a definite learning curve
The logistics and processes when coordinating a study of this type are extremely complicated. However, NASH is a primary indicator for Cerba Research, so the quality processes and experience were key factors for successful conduct in an international trial this large.
The team established comprehensive operational workflows and internal monitoring with an emphasis on the primary endpoint liver biopsy.
Cerba’s worldwide affiliates’ regional project managers assisted local needs and helped preserve consistency and standardization at a global scale.
Cerba Research’s proactive site training and monitoring strengthened sites’ compliance with protocol expectations, including quality slide production and proper blinding practices. Moreover, a coordinated global study database allowed study teams around the world to access all trial data via Cerba’s online portal.
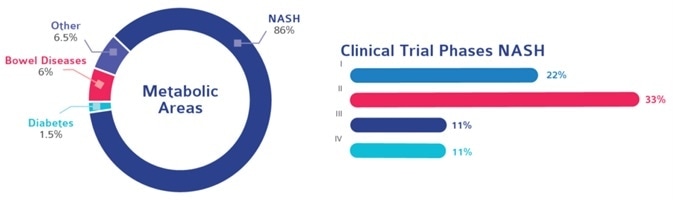
Image Credit: Cerba Research
Cerba, the central lab centered around you
Cerba Research believes customer-focused project management is a vital part of running a smooth trial. A great deal of collaboration was needed to manage each component of this project. Throughout, the company’s SMEs and PMs communicated freely and proactively with the client to optimize services and processes.
Typically, the Cerba Research team has the capacity to assist optimal biomarker selection specific to the study requirements, molecule and budget. Cerba can also facilitate the compliance of the proposed protocol in line with ever-changing FDA, EMA or other regulatory body suggestions or regulations.
Cerba successfully partners with CROs by offering them NASH expertise. For example, a joint CRO-lab offering, integrated seamlessly, can be mutually profitable while delivering optimal results and efficiencies for sponsors.
On the other hand, NASH studies are notorious for high screen-failure rates and inflating costs. During the trial, Cerba closely monitors the budget by tracking expenditures and immediately highlighting any outliers or excesses to proactively address potential overages.
Learnings from this trial help us anticipate future needs
Change is to be expected in clinical research, specifically in indications as recent as NASH. Cerba Research has used the relevant insights from this phase III NASH trial to design a better range of offerings that increases client efficiency while addressing any imminent regulatory updates.
Cerba was particularly inspired by the latest requirement for multiple biopsy readers and the expected advancement to digitalization and evaluation by AI.
Cerba Research’s liver biopsy services will soon or already include:
- An app to improve processes
- Digitalized slides
- Integrated platforms
- Next-generation image analysis
Other unique offerings are:
- BioKortex, a patient-centric recruitment solution that utilizes the enormous Cerba HealthCare patient database to reduce costs and save sponsors time through customized digital tools, patient-enriched data and partnerships with strong medical laboratory networks.
- Metabolomics, whereby analysis of a mass spec profile of serum metabolites is conducted against a NASH/steatosis database. This method can be utilized to assess short interval responses to therapy, evaluate a drug’s mechanism of action or customize a companion diagnostic.
- Richer resources for site training and site performance management in combination with a site performance database to determine the likelihood of success.
Cerba anticipates that these new options will deliver improved value for its clients.
Cerba means certainty
Working across a global patient base can be quite a challenge. Yet, across continents to rural villages, through language, customs barriers and regulatory requirements, Cerba gets the job done.
Through close collaboration, Cerba supplied a midsize biotech company with global access to industry-leading laboratories, scientific experts and skilled project management needed to successfully support this challenging NASH trial.
As part of its detailed lab support, Cerba processed in excess of 5000 liver biopsies and had them certified by a KOL.
When partnering with Cerba Research for worldwide central laboratory services, the focus is always centered on the clinical trial.
Partners can rely on over 35 years of experience, industry-leading laboratories that cross five continents and a six-week standard startup time. The experience is excellent and seamlessly efficient in central lab support for clinical trials.
About Cerba Research
For over 35 years, Cerba Research has been setting the industry standard for exemplary clinical trial conduct. Today, across five continents, with a focus on precision medicine, we are changing the paradigm of the central lab’s role in complex clinical research.
From protocol inception through development and to market, our passionate experts deliver the highest quality specialized and personalized laboratory and diagnostic solutions. Partner with us for the most efficient strategy to actualize your biotech and pharmaceutical products sooner and improve the lives of patients worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.