The untreatable disease multiple myeloma (MM) is characterized by the existence of malignant plasma cells, which secrete significant levels of a monoclonal immunoglobulin protein (M-protein).1,2
The International Myeloma Working Group (IMWG) has confirmed a range of criteria for clinical response to treatment in MM. This includes alterations in serum/urine M-protein levels via immunofixation electrophoresis (IFE) and serum protein electrophoresis (SPE), percentage of bone marrow plasma cells and free light chain (FLC) ratios.3,4,5
According to the IMWG criteria, for a patient to be considered to have a complete response (CR), IFE and SPE must confirm that the serum and urine are negative for M-protein, and bone marrow plasma cells must be ≤ 5%.
CR in serum FLC-only patients is considered a normal FLC ratio, plus the other criteria necessary in classifying a CR.4
The more robust, in-depth classification of stringent complete response (sCR) requires all criteria for CR to be met, as well as a normal FLC ratio and a lack of clonal plasma cells in the bone marrow (measured using immunohistochemistry or 2- to 4-color flow cytometry).
Treatment of MM has continued to develop in line with the introduction of therapeutic monoclonal antibodies (mAbs).6,7,8 SPE and IFE assays can be used to quantify and characterize the clonal nature of immunoglobulins, but they may suffer from interference due to the use of therapeutic mAbsn.9,10
Trials with spiked samples have shown that mAbs can be consistently detected via SPE and IFE, down to as little as 0.1 g/L.10 There have been reports of interference on serum IFE from treated patients with a number of mAbs, including siltuximab, ofatumumab and daratumumab,1,9,10 with similar interference also noted when using elotuzumab.7,11
As IMWG’s criteria for CR require there to be no detectable M-protein (confirmed via IFE and SPE),3 antibody interference may present a clinically important factor in terms of the assessment of response to treatment, potentially even leading to an underestimation of CR rates for mAb therapies.
Therapeutic mAbs are increasingly employed in myeloma, meaning that methods are required to evaluate clinical responses – most notably CR/sCR – to ascertain the impact of this potential interference.
The human IgG1κ mAb daratumumab displays high-affinity binding to a distinct CD38 epitope. This induces tumor cell death through a variety of mechanisms, such as antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, antibody-dependent cellular phagocytosis and the induction of apoptosis.12,13,14,15
Subpopulations of regulatory B cells, regulatory T cells, and myeloid-derived suppressor cells with high CD38 expression have also been found to be susceptible to daratumumab.16
It has also been noted that cytotoxic T cell activation, expansion, and increased T cell clonality occur following monotherapy treatment in relapsed or refractory disease, potentially indicating an immunomodulatory role for daratumumab in MM.16
In GEN501, it was found that a phase 1/2 study of patients with refractory or relapsed MM exhibited good tolerance to daratumumab monotherapy. A total of 36% of these patients receiving daratumumab at 16 mg/kg were determined to exhibit at least a partial response (PR) or better.6
The phase 2 study SIRIUS investigated daratumumab in patients with at least three lines of prior therapy or double refractory MM.8 The overall response rate (ORR) of the treatment was 29%, with responses deepening with ongoing treatment.
The average total survival rate in these severely pre-treated patients (median of five prior lines of treatment) was 17.5 months (95% confidence interval, 13.7–not estimable).8
These studies have led to daratumumab being approved in the United States for treating patients with MM who have received a minimum of three lines of prior therapy - where this therapy involved an immunomodulatory drug (IMiD) and a proteasome inhibitor (PI) – and/or are double refractory to an IMiD and a PI.17
Investigations are presently taking place into daratumumab as part of phase 3 clinical studies. These studies see daratumumab used in conjunction with other therapeutic agents in patients with MM.
Daratumumab’s recommended dosing schedule is 16 mg/kg weekly for eight weeks, then every two weeks for 16 weeks, and every four weeks following that. In line with this schedule, daratumumab reaches peak serum concentrations of approximately 915 μg/mL (0.915 g/L) by the end of the weekly dosing part of the schedule.18
Once it reaches these levels, it is easily detectable via the majority of SPE/IFE assays.1
Since it is a human IgGκ immunoglobulin, there is a chance that daratumumab could be erroneously detected by IFE, causing this to be mistakenly identified as a myeloma-associated M-protein, therefore interfering with the defined response criteria.19
The daratumumab-specific immunofixation electrophoresis reflex assay (DIRA) was developed to better distinguish daratumumab from endogenous M-protein in serum IFE. This assay is able to confirm suspected daratumumab interference, enabling the effective separation of daratumumab bands from residual endogenous M-protein.
DIRA employs an anti-daratumumab antibody which is able to bind daratumumab and change its migration on IFE. This article details a process of validation in terms of DIRA’s use in clinical trial testing. The study involved determining the assay’s specificity, the limit of sensitivity and reproducibility.
The assay is currently used in clinical trials that are looking to distinguish daratumumab from endogenous M-protein by IFE. This work has prompted further clinical response assessments intending to confirm CRs in myeloma patients treated with daratumumab.
Materials and methods
Serum sample collection
The researchers acquired human serum samples from patients with MM or healthy donors via a combination of a commercial supplier (Bioreclamation, Westbury, NY, USA) and daratumumab-treated patients (n= 33).
Serum samples from clinical trials which were either using daratumumab as monotherapy (GEN501 and SIRIUS) or as a combination therapy with lenalidomide in an ongoing study (GEN503; ClinicalTrials.gov Identifier: NCT01615029) were acquired in 2.5 or 8.5 mL serum separator tubes (Becton Dickinson, Franklin Lakes, NJ, USA).
These tubes were centrifuged at 1300–2000 ×g for 10–15 minutes after being left for 30 minutes at room temperature. This was done to allow the blood to clot and cool fully.
Serum samples were collected, frozen and shipped to a central laboratory (BARC, Ghent, Belgium) to undergo further DIRA testing, or SPE and IFE.
Patients that presented with low-level (< 5 g/L) or negative SPE but exhibited repeated positive IgGκ IFE were concluded to be experiencing potential daratumumab interference.
These specific patients were used for validation and DIRA testing, and samples were based on suspected interference as opposed to predetermined time points.
These clinical trials have been approved by independent Institutional Review Boards at study sites in line with the Declaration of Helsinki. They are fully compliant with Good Clinical Practices, and every patient involved provided written informed consent.
Anti-daratumumab antibody
A hybridoma cell line (Genmab, Utrecht, The Netherlands) was used to produce a murine anti-daratumumab antibody clone (5–3–9–4) (Johnson & Johnson, New Brunswick, NJ, USA).
Supernatants acquired from cultured cells were concentrated via tangential flow filtration (Millipore, Billerica, MA, USA). These were purified using MabSelect-Sure (GE Healthcare, Marlborough, MA, USA) before being dialyzed into Dulbecco’s phosphate-buffered saline, pH 7.2 (Life Technologies, Grand Island, NY, USA).
IFE and SPE
Maxikit Hydragel 4IF or 9IF (Sebia, Norcross, GA, USA) was used to perform immunofixations on semi-automatic Hydrasys or Hydrasys 2. SPE was done on Capillarys using the Capillarys Protein 6 kit (both from Sebia). The IFE and SPE processes were undertaken in line with the manufacturer’s specifications.
DIRA
The DIRA testing process saw anti-daratumumab or saline being spiked into baseline or daratumumab-treated patient serum. This was then incubated at room temperature for a total of 15 minutes before this was separated using electrophoresis in line with the standard IFE methods previously discussed.
One lane of each daratumumab-treated patient’s serum and baseline was fixed as a reference. Next, anti-human, anti IgG or κ (Sebia) antisera were administered in order to detect heavy and light chains.
Once electrophoresis and staining had been completed, the gels were assessed for a number of factors:
- Migration of control daratumumab with anti-daratumumab
- A notable lack of migration of baseline M-protein with added anti-daratumumab
- A shift in the putative daratumumab band’s migration pattern in relation to the daratumumab control in daratumumab-treated serum samples
- The absence or presence of a non-daratumumab M-protein band
A DIRA-negative result was considered to be an absence of remaining disease M-protein, while a DIRA-positive result was considered to be the presence of remaining disease M-protein.
Limit of sensitivity
A total of 10 commercial MM samples were spiked with 0.25, 0.5 and 1.0 g/L daratumumab. This was done both with and without anti-daratumumab in a 1:1 ratio in order to ascertain the reproducibility and effectiveness of anti-daratumumab in terms of shifting daratumumab bands.
Next, another 10 MM serum samples and 10 normal human serum (NHS) samples were spiked using a broader range of clinically-relevant daratumumab concentrations (0, 0.1, 0.2, 0.25 and 0.5 g/L).
These were completed with and without anti-daratumumab in a 1:1 ratio. The results were evaluated by two independent reviewers.
The limit of sensitivity was specified as the lowest level of daratumumab that is detectable by at least one parameter; for example, daratumumab IgG, daratumumab κ anti-daratumumab complex IgG, daratumumab κ, or daratumumab + anti-daratumumab κ by IFE; daratumumab or daratumumab + anti-daratumumab by SPE.
This limit of sensitivity was employed in all samples tested.
Specificity
In order to confirm and illustrate that the anti-daratumumab antibody had no impact on shifting endogenous M-protein migration, a series of commercially-available serum samples from patients with MM (n=51) were spiked with daratumumab, anti-daratumumab, or daratumumab + anti-daratumumab (0.5 g/L and 1 g/L; 1:1 ratio) before being analyzed using IFE.
A subset of these samples (n=35) was used to assess fixed concentrations of 1 g/L anti-daratumumab and 0.5 g/L daratumumab. Gels were evaluated to identify shifts in daratumumab, with no corresponding shift in M-protein with anti-daratumumab in isolation.
In each of the DIRA assays, control serum samples taken from patients before treatment with daratumumab were spiked with anti-daratumumab. These samples were assessed to identify any shift of endogenous M-protein on IFE.
Reproducibility
A total of three independent runs of 10 commercial samples were spiked with 0.25, 0.5 and 1 g/L daratumumab, with a further ten samples from daratumumab-treated patients with M-protein ≤5 g/L, by SPE. All of these runs were conducted using DIRA.
Two independent reviewers evaluated the results of these tests to ascertain their reproducibility. Evaluations from the reviewers’ were standardized according to predefined assessment criteria. Table 1 displays these criteria and the reviewers’ responses.
Table 1. Concordance of reviewer assessments of the same sample across multiple experiments based on predefined acceptance criteria. Source: Cerba Research
| test |
Lane |
Run 1 |
Run 2 |
Run 3 |
| Reviewer 1 |
| Migration of Dara + anti-Dara in control? |
4 vs. 3 |
Y |
Y |
Y |
| Migration of endogenous M-protein at baseline? |
6 and 10 |
N |
N |
N |
Migration of Dara in ≥PR due to the disappearance of Dara (DD)
or the appearance of Dara + anti-Dara complex (AC)? |
8 vs. 7 and 12 vs. 11 |
Y
DD+AC |
Y
DD+AC |
Y
DD+AC |
| Presence of M-protein after migration of Dara? |
8 and 12 |
N |
N |
N |
| M-protein (M) or Dara (D)? |
|
D |
D |
D |
| Conclusion |
|
Negative |
Negative |
Negative |
| Reviewer 2 |
| Migration of Dara + anti-Dara in control? |
4 vs. 3 |
Y |
Y |
Y |
| Migration of endogenous M-protein at baseline? |
6 and 10 |
N |
N |
N |
Migration of Dara in ≥PR due to the disappearance of Dara (DD)
or the appearance of Dara + anti-Dara complex (AC)? |
8 vs. 7 and 12 vs. 11 |
Y
DD+AC |
Y
DD+AC |
Y
DD+AC |
| Presence of M-protein after migration of Dara? |
8 and 12 |
N |
N |
N |
| M-protein (M) or Dara (D)? |
|
D |
D |
D |
| Conclusion |
|
Negative |
Negative |
Negative |
Dara, daratumumab; Y, yes; N, no; PR, partial response.
Inter-operator and inter-day reproducibility was assessed using three commercial MM samples on three separate days and two operators. These results were interpreted by two independent reviewers.
Results
Shifting daratumumab with anti-daratumumab
To determine whether it was possible to detect a shift in daratumumab using SPE and IFE, spiking experiments were conducted with different concentrations of daratumumab, with or without anti-daratumumab added to myeloma serum or NHS. These were then analyzed using SPE or IFE.
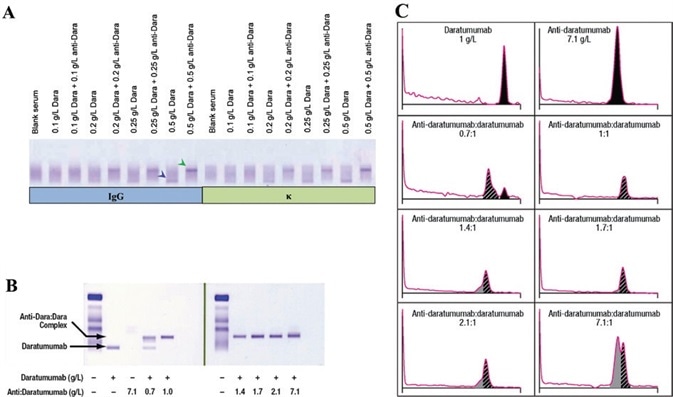
Figure 1. Daratumumab can be identified on IFE/SPE and can be shifted with anti-daratumumab. Daratumumab can be detected by IFE (A), anti-daratumumab antibody can bind and shift daratumumab migration pattern on IFE (B); 1:1 ratios of daratumumab:anti-daratumumab are enough to completely shift daratumumab on IFE. Similarly, on SPE, a 1:1 ratio of daratumumab:anti-daratumumab was able to completely shift daratumumab (C). Daratumumab and daratumumab:anti-daratumumab complex are indicated by the blue and green arrows, respectively. IFE, immunofixation electrophoresis; SPE, serum protein electrophoresis; Dara, daratumumab. Image Credit: Cerba Research
In every sample tested, it was possible to determine that daratumumab was effectively detected and shifted with anti-daratumumab (Figure 1A - data not shown).
To evaluate the amount of anti-daratumumab needed to completely shift daratumumab via IFE and SPE, different ratios of anti-daratumumab were spiked into the serum, which contained 1 g/L daratumumab - the maximum expected concentration in patient serum following weekly dosing.15
These tests confirmed that a 1:1 ratio of daratumumab:anti-daratumumab or excess anti-daratumumab entirely shifted daratumumab on IFE (Figure 1B). It was also determined that excess mouse anti-daratumumab was not detected by human antiserum.
By leveraging densitometry of SPE lanes, it was possible to show that a 1:1 ratio of daratumumab:anti-daratumumab was required to completely shift daratumumab.
In this example, excess daratumumab or anti-daratumumab were also visualized as protein peaks because SPE is unable to discern total protein from human or mouse antibodies (Figure 1C). IFE was used to develop DIRA due to its improved sensitivity and its specificity for human antibodies.
Distinguishing daratumumab from endogenous M-protein
Patients enrolled in daratumumab clinical studies typically exhibited a residual IgGκ band or a faint IgGκ band appearing over time when this was investigated on IFE.
Daratumumab interference was suspected in these instances, potentially masking CRs. DIRA was developed to better distinguish daratumumab from endogenous M-protein where patients presented with low measurable M-protein by SPE ( ≤5 g/L) and IgGκ band by IFE.
Exploratory analyses were performed using samples which presented a higher range of SPE in order to help refine criteria for assay implementation and validation. Figure 2 displays a summary schematic of controls, samples and loading in a standard DIRA.
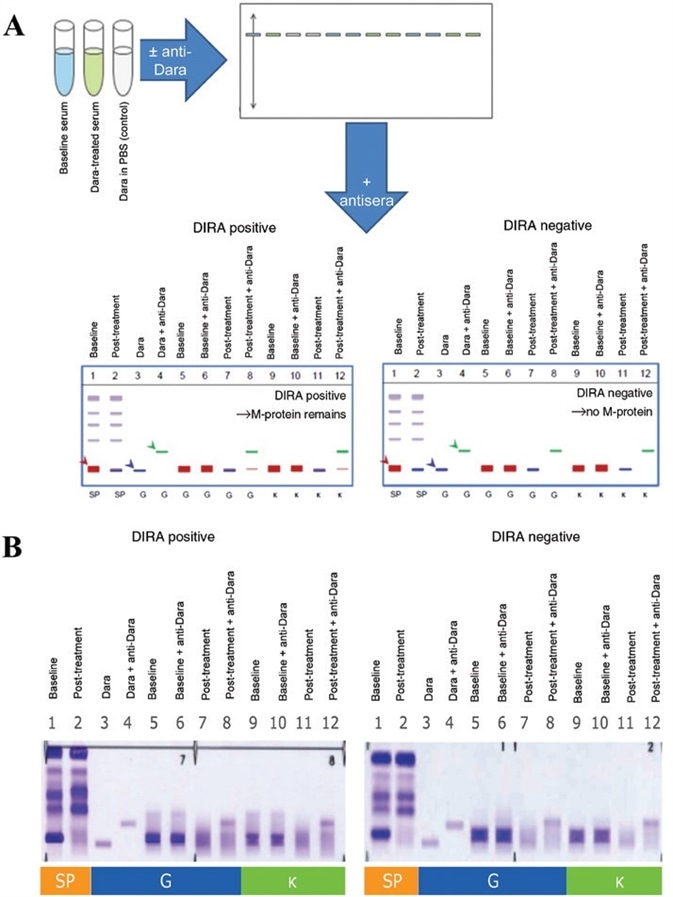
Figure 2. Daratumumab-specific IFE reflex assay. Baseline (prior to treatment) serum samples are run ± anti-daratumumab next to serum samples from a post-treatment time point with suspected daratumumab interference, ± anti-daratumumab, to determine whether the remaining M-protein band shifts completely with antidaratumumab. Both IgG and κ antisera are used for staining and fixation (A). DIRA positive, similar to IFE positive, indicates that endogenous M-protein (in red, and indicated by a red arrow in lane 1) remains. DIRA negative, similar to IFE negative, indicates that only daratumumab (in blue, and indicated by a blue arrow in lane 3) is remaining and endogenous M-protein is no longer detected (A). The DIRA template utilized daratumumab ± anti-daratumumab as controls for migration of the therapeutic antibody and the daratumumab-anti-daratumumab shifted complexes (in green, and indicated by a green arrow in lane 4). In patient samples, baseline and post-treatment serum ± anti-daratumumab were compared to determine whether M-protein remained after shifting daratumumab (B). DIRA-positive results showed M-protein, whereas DIRA-negative results showed only a shift in daratumumab but no remaining M-protein (lanes 8 and 12). IFE, immunofixation electrophoresis; M-protein, monoclonal immunoglobulin protein; DIRA, daratumumab-specific immunofixation electrophoresis reflex assay; Dara, daratumumab; PBS, phosphate buffered saline; SP, total serum protein fix; G, IgG antisera; κ, kappa antisera. Image Credit: Cerba Research
DIRA is used to assess patient samples prior to (baseline) and following treatment in cases where daratumumab interference is suspected. DIRA necessitates the use of 12 sample lanes, employing a protein fixative and two antisera: IgG and κ (Figure 2A).
Lanes 1 and 2 are made up of baseline and post-treatment samples with total protein fixative. These lanes show the migration patterns of every serum protein at both baseline and post-treatment.
Lanes 3 and 4 are controls that control daratumumab and daratumumab + anti-daratumumab in saline, respectively.
Lanes 5 and 6 feature anti-IgG antisera and include the baseline sample alone and the baseline sample with anti-daratumumab, correspondingly. These lanes are included to facilitate the characterization of endogenous M-protein migration and to confirm that anti-daratumumab alone does not affect endogenous M-protein.
Lanes 7 and 8 also feature anti-IgG antisera. These lanes include the post-treatment sample alone and the post-treatment sample with anti-daratumumab, respectively. These lanes are included to characterize daratumumab and ascertain whether or not disease M-protein remains.
Suppose the whole remaining band shifts with the addition of anti-daratumumab, confirming that endogenous M-protein is absent and only daratumumab remains. In this case, this result is considered to be DIRA negative. This is similar to a typical IFE-negative result (Figure 2B).
In instances where the band only partially shifts, confirming that endogenous M-protein remains, then this result is considered to be DIRA positive. This is similar to a standard IFE-positive result (Figure 2B).
Lanes 9 to 12 feature identical samples to lanes 5 through 8, but these are instead probed with anti-κ antisera.
Validation of DIRA
Evaluations were undertaken on the sensitivity, specificity and reproducibility of DIRA. This was done with both commercial and daratumumab-treated myeloma serum samples in order to facilitate robust clinical validation.
Determination of sensitivity was done via an evaluation of 10 myeloma and 10 NHS samples that were spiked with a range of daratumumab + anti-daratumumab via SPE and IFE.
Daratumumab or the daratumumab-anti-daratumumab complex’s potential to comigrate with M-protein with either IgG or κ antisera, sensitivity was defined by detecting this with at least one parameter (daratumumab or daratumumab + anti-daratumumab complex with IgG or κ by IFE; daratumumab or daratumumab + anti-daratumumab complex by SPE).
The sensitivity per sample was identified by determining the lowest level of daratumumab that could be detected by any of the parameters above.
DIRA’s sensitivity in myeloma serum samples was found to be 90% for 0.1 g/L daratumumab and 100% for 0.2 g/L by IFE.
In NHS, DIRA’s sensitivity by IFE was found to be 80% for 0.1 g/L daratumumab and 100% for 0.2 g/L. By SPE, however, DIRA’s sensitivity was found to be 30% for 0.1 g/L and 100% for 0.2 g/L in MM serum and 100% at 0.2 g/L in NHS.
The sensitivity of DIRA for daratumumab was, therefore, determined to be ~0.2 g/L. MM patients are generally immunosuppressed, meaning that issues caused by background polyclonal interference have been negligible thus far.
It was impossible to reliably identify residual daratumumab below 0.2 g/L in spiked NHS samples.
IFE - and therefore DIRA - is not a quantitative assay, but it was possible to determine that the lower range of sensitivity demonstrated daratumumab could be detected and that DIRA was sufficiently functional within the range of expected serum concentrations in treated patients.
DIRA’s specificity is dependent on the specificity of the anti-daratumumab. DIRA, therefore, includes control lanes which feature baseline serum samples spiked either with or without anti-daratumumab (Figure 2, Lanes 5 and 6).
When assessing commercial samples spiked with 0.5 or 1 g/L daratumumab, it was possible to consistently shift the antibody by anti-daratumumab at both concentrations (51 of 51 [100%]).
It was also noted that there was no noticeable shift in M-protein in any sample with the addition of only anti-daratumumab. When anti-daratumumab alone was spiked into the serum, it was possible to observe a weak polyclonal smear in the lanes with IgG antisera in 4 of 51 (8%) samples.
This did not interfere with the interpretation of DIRA, however, because the band corresponding to the daratumumab:anti-daratumumab complex was clearly visible, and there was no notable smear in samples when daratumumab was present.
Experienced reviewers are typically able to identify the faint residual band in DIRA assays, but this may pose challenges to identification in Figure 2B. This issue is common and recognized with faint bands on agarose gels because scanned gels do not have the same resolution or detail as their physical counterparts.20
Anti-daratumumab, therefore, is considered to be highly specific for daratumumab. Future research will examine the anti-daratumumab antibody’s specificity, alongside DIRA’s false-negative and false-positive rates. This will take the form of a randomized, phase 3 clinical study of daratumumab versus control.
DIRA’s reproducibility was evaluated by running the assay on daratumumab-treated patient samples in triplicate. Results were found to be consistent across three independent experiments, and this was the case in every daratumumab-treated patient sample (10/10).
In order to evaluate inter-day and inter-operator reproducibility, DIRA was performed on commercial samples spiked with daratumumab + anti-daratumumab on three separate days by two different operators. The results of these tests were also evaluated by two independent reviewers.
It was noted that concordance among reviews was achieved in 100% of assays. Table 1 displays the reviewers’ responses to a series of predetermined assessment criteria for a single patient sample over the three separate experiments.
DIRA Plus
Samples from 14 daratumumab-treated patients were tested with increased concentrations of anti-daratumumab.
This modification - known as ‘DIRA Plus’ (Figure 3) - was put in place to confirm that 1 g/L anti-daratumumab was sufficient enough to shift daratumumab in patient serum samples for which daratumumab concentration data is not available or in instances where SPE measurements were found to be higher than the average range of daratumumab concentrations.
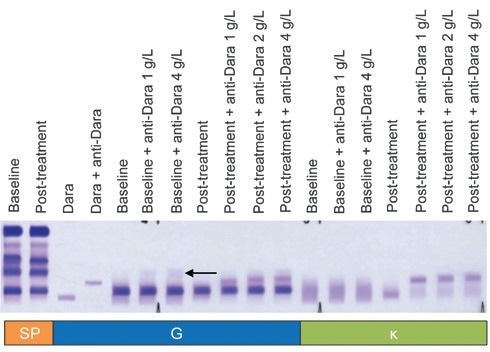
Figure 3. DIRA Plus for the evaluation of patients with serum concentrations of daratumumab above the normal range. One gram per liter of anti-daratumumab was sufficient to migrate daratumumab in all samples (A). Higher concentrations of anti-daratumumab added to baseline serum (1 g/L and 4 g/L) resulted in the appearance of a faint, polyclonal smear (arrow) with IgG antisera. DIRA, daratumumab-specific immunofixation electrophoresis reflex assay; Dara, daratumumab; SP, total serum protein fix; G, IgG antisera; κ, kappa antisera. Image Credit: Cerba Research
Anti-daratumumab concentrations of 1–4 g/L were used for these assays, and in every case (14 of 14 samples), 1 g/L of anti-daratumumab was found to be sufficient to interpret DIRA.
It was also determined that anti-daratumumab concentrations ≥1 g/L prompted the appearance of a weak, polyclonal smear, though there was no other change in the assay result when compared to the standard concentration of 1 g/L.
These findings confirm that the use of anti-daratumumab concentrations >1 g/L is neither necessary nor advisable.
Incorporating DIRA into clinical testing
An operational ‘algorithm’ for triggering DIRA was devised to automate the initiation of DIRA – an important tool when conducting phase 3 clinical trials with significant numbers of daratumumab-treated patients.
The algorithm specifies that in cases where only IgGκ M-protein is detected on IFE and urine, and FLC results are found to be normal, DIRA testing should be conducted for patients exhibiting M-protein levels ≤ 2 g/L by SPE on two consecutive visits (Figure 4).
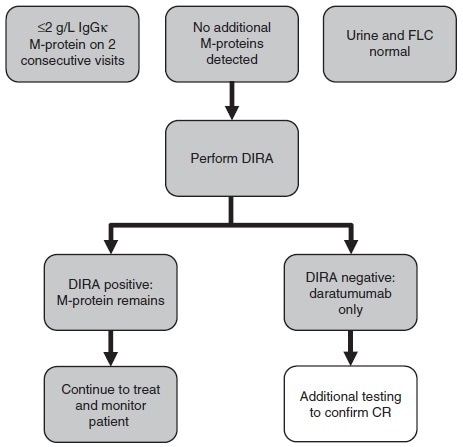
Figure 4. Testing algorithm to implement DIRA for clinical response assessment. Samples from a patient with ≤2 g/L IgGκ M-protein on two consecutive visits, with normal urine and FLC and without additional M-protein, are good candidates for DIRA. For patients with DIRAnegative samples, additional testing to confirm a putative CR is warranted. DIRA, daratumumab-specific immunofixation electrophoresis reflex assay; M-protein, monoclonal immunoglobulin protein; FLC, free light chain; CR, complete response. Image Credit: Cerba Research
Should the results be found to be DIRA negative, patients should undergo further testing to confirm CR, for example, bone marrow evaluation of plasma cells.
Should the results be found to be DIRA positive - indicating remaining disease M-protein – then there will be no need for further testing - disease monitoring should continue (Figure 4).
Discussion
The human anti-CD38 mAb daratumumab has been shown to offer robust clinical efficacy in refractory and relapsed myeloma, including CRs in certain patients.
Since it is a monoclonal immunoglobulin, daratumumab can be detected via SPE and IFE assays employed in the monitoring and characterization of an endogenous immunoglobulin protein.
When implemented at the suggested 16 mg/kg dose and schedule, it was determined that the mean (±standard deviation) maximum trough daratumumab concentration was 0.573±0.331 g/L. At this concentration, the daratumumab has the potential to interfere with the interpretation of the SPE and IFE assays (data on file).
The latest IMWG criteria for a CR include the use of negative serum, urine protein electrophoresis and IFE – an approach which is not viable when daratumumab is present at concentrations within the typical therapeutic range.
DIRA has been developed, validated and implemented to address this issue, making it possible to distinguish daratumumab from myeloma M-protein.
DIRA employs an extremely specific anti-daratumumab antibody to bind daratumumab and effectively shift its migration on IFE gels. Patients presenting with a single IgG κ band that is entirely shifted by DIRA are understood to have no remaining M-protein (DIRA negative).
These patients would therefore be candidates for further IMWG-required confirmatory testing to confirm whether criteria for CR/sCR (as defined by the IMWG) are met. This testing could involve bone marrow assessment for plasma cells.
Patients who present remaining endogenous M-protein on DIRA are regarded as DIRA positive, and disease monitoring continues.
DIRA was found to be highly sensitive, specific and reproducible in the commercial samples spiked with daratumumab and in the clinical samples from daratumumab-treated patients.
It was determined that the presence – at normal or even excess levels – of anti-daratumumab had no impact on the detection or migration of endogenous M-proteins.
When daratumumab was absent, it was noted that a weak polyclonal smear was present in IgG antisera lanes. This was the case in 4 of the 51 samples when anti-daratumumab was added, but it was still possible to clearly identify the daratumumab:anti-daratumumab complex via visual inspection.
This did not interfere with the interpretation of DIRA – it was still possible to detect daratumumab by at least one parameter.
DIRA’s limit of sensitivity was confirmed to be 0.2 g/L in serum from patients with myeloma. There is a high likelihood of interference with M-protein at this concentration and above.
Trough daratumumab concentrations throughout the weekly and every 2 weeks dosing periods were generally higher than DIRA’s sensitivity and could lead to daratumumab detection by IFE.
Daratumumab trough concentrations during every 4 weeks dosing could fall below the DIRA sensitivity, however, meaning there would be no interference with M-protein monitoring during this window.
It was also possible to modify DIRA to accommodate patients with higher-than-average serum concentrations. This method – referred to as ‘DIRA Plus’ – increases the amount of anti-daratumumab employed, though it was noted that assay reliability became lower with increasing anti-daratumumab concentrations <1 g/L.
To assess reproducibility, two independent reviewers scored all DIRA tests. The reviewers’ assessments were consistently in agreement, meaning that there was no need to employ a third reviewer.
Reproducibility tests were undertaken with a series of 10 samples, with results for individual samples proving to be consistent across several repetitions. These findings illustrate the robustness of DIRA testing, successfully highlighting its high sensitivity, specificity and reproducibility.
DIRA does have a number of limitations despite its advantages.
DIRA is not a quantitative method, meaning that it must be interpreted by a trained operator. While it is not common in myeloma, high polyclonal background signals could lead to challenges in assessing responses in some patients, resulting in false interpretations.
DIRA is only highly specific to daratumumab, meaning that it is not possible to resolve responses in patients receiving other antibodies by using DIRA.
In these instances, it is necessary to use different methods to address antibody interference, with techniques such as mass spectrometry essential when working with patients receiving combinations of antibodies or where there is a need for quantitative testing.
DIRA has the potential to be a vital tool in determining response in daratumumab clinical studies. This is especially the case when working with patients with IgGκ M-protein.
Patients presenting with non-IgGκ endogenous serum M-proteins – for example, FLC, urine, IgA κ or λ or IgGλ M-proteins – that were positive for IgG κ by IFE were found to be easily detectable using DIRA. To meet current IMWG criteria, however, these must also be assessed to show that only daratumumab remained.
During phase 2 studies, it was noted that an IgGκ band frequently appeared in SPE/IFE over the course of daratumumab treatment in patients. This was the case, even when the patients had been originally classified as having either non-IgGκ myeloma such as IgA, IgM, IgE myeloma or light-chain-only myeloma.
It is believed that this band is a result of daratumumab interference as opposed to a new plasma cell clone which is secreting an IgG monoclonal M-protein.
The original myeloma clone was found to be IgA or light chain only in approximately 24% and 11% of patients in the general myeloma population, respectively.21 Daratumumab was easily identified with DIRA in these circumstances, allowing a lack of endogenous M-protein to be confirmed.
It is important to note, however, that 60% of patients with MM have IgG M-protein,21 meaning it can be difficult to distinguish between daratumumab and endogenous M-protein for those patients with IgGκ.
The study showed that the most challenging cases to interpret were those where the migration of daratumumab entirely overlapped with M-protein and the whole band failed to shift with anti-daratumumab.
Following the operational algorithm and waiting until M-protein measurements on SPE were lower helped to reduce the quantity of these cases.
Recently, IMWG released a clarification in order to better address antibody interference. The updated guidelines required just the original myeloma clone(s) to be undetectable by SPE/IFE.22 DIRA is still able to distinguish IgGκ clones from daratumumab, however.
The ongoing development and validation of DIRA provide a viable solution for the mitigation of daratumumab interference in IFE, helping to improve daratumumab-treated patients’ clinical response assessment.
There was no need to address the issue of mAb assay interference until recently due to the historically modest success of mAbs for the treatment of MM in the clinic.
Phase 1 and 2 studies investigating the use of daratumumab as a monotherapy have generated deep responses, which have included CRs and sCRs.6,8 These results have prompted the pressing need to establish a reliable means of distinguishing M-protein from daratumumab.
As myeloma therapy continues to integrate other mAb therapeutics, additional approaches to the mitigation of antibody interference on SPE/IFE will become necessary.
Alternative approaches could see the incorporation of minimal residual disease (MRD) detection into more formal clinical criteria for CR and sCR.
MRD detection by 8- to 10-color flow cytometry could also be vulnerable to antibody interference, but a solution may lie in the use of standardized approaches using non-competing antibodies. It may also be prudent to employ methods such as polymerase chain reaction or next-generation sequencing.
Conclusions
DIRA continues to represent an effective test with high sensitivity, specificity and reproducibility. This method is therefore ideal for distinguishing endogenous M-protein from daratumumab.
DIRA is already being utilized in daratumumab clinical trials looking to understand whether or not patients with outcomes of very good PR would benefit from confirmatory assessments to enable characterization of CR. These ongoing studies are expected to offer further functional validation of DIRA as a clinical tool.
References
- Axel AE, McCudden CR, Xie H, Hall BM, Sasser AK. Development of clinical assay to mitigate daratumumab, an IgG1k monoclonal antibody, interference with serum immunofixation (IFE), and clinical assessment of M-protein response in multiple myeloma. Cancer Res 2014;74(Suppl. 19). Abstract 2563.
- Zent CS, Wilson CS, Tricot G, Jagannath S, Siegel D, Desikan KR, et al. Oligoclonal protein bands and Ig isotype switching in multiple myeloma treated with high-dose therapy and hematopoietic cell transplantation. Blood 1998;91:3518–23.
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia 2006;20:1467–73.
- Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011;117:4691–5.
- Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009;23:3–9.
- Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015;373:1207–19.
- Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015;373:621–31.
- Lonial S, Weiss B, Usmani S, Singhal S, Chari A, Bahlis N, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016 Jan 6. pii: S0140-6736(15)01120-4. doi: 10.1016/S0140-6736(15)01120-4. [Epub ahead of print].
- Genzen JR, Kawaguchi KR, Furman RR. Detection of a monoclonal antibody therapy (ofatumumab) by serum protein and immunofixation electrophoresis. Br J Haematol 2011;155:123–5.
- McCudden CR, Voorhees PM, Hainsworth SA, Whinna HC, Chapman JF, Hammett-Stabler CA, et al. Interference of monoclonal antibody therapies with serum protein electrophoresis tests. Clin Chem 2010;56:1897–9.
- Dimopoulos M, Lonial S, Casado LF, Golightly M, Doyen C, Shelat S, et al. Elotuzumab: serum protein electrophoresis and immunofixation interference with clinical assessment of M-protein response in relapsed/refractory multiple myeloma (RRMM). Poster presented at: 15th International Myeloma Work- shop; September 23–26, 2015; Rome, Italy.
- de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 2011;186:1840–8.
- Jansen JH, Boross P, Overdijk MB, Lammerts van Bueren JJ, Parren PW, Leusen HH. Daratumumab, a human CD38 antibody induces apoptosis of myeloma tumor cells via Fc receptor- mediated crosslinking. Blood 2012;120. Abstract 2974.
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Abstract presented at: 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, CA. Abstract 3474.
- Overdijk MB, Verploegen S, Bogels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015;7:311–21.
- Krejcik J, Casneuf T, Nijhof I, Verbist B, Bald J, Plesner T, et al. Immunomodulatory effects and adaptive immune response to daratumumab in multiple myeloma. Blood 2015;126. Abstract 3037.
- McKeage K. Daratumumab: first global approval. Drugs 2016;76:275–81.
- McCudden C, Axel A, Slaets D, Frans S, Bald J, Schecter JM, et al. Assessing clinical response in multiple myeloma (MM) patients treated with monoclonal antibodies (mAbs): validation of a daratumumab IFE reflex assay (DIRA) to distinguish malignant M-protein from therapeutic antibody. J Clin Oncol 2015;33. Abstract 8590.
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–48.
- Bender LM, Cotten SW, Fedoriw Y, Willis MS, McCudden CR. Evaluation of digital images for identification and characterization of monoclonal immunoglobulins by immunofixation. Clin Biochem 2013;46:255–8.
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23:3412–20.
- Durie BG, Miguel JF, Blade J, Rajkumar SV. Clarification of the definition of complete response in multiple myeloma. Leukemia 2015;29:2416–7.
Acknowledgements
Produced from materials originally authored by Christopher McCuddena, Amy E. Axela, Dominique Slaets, Thomas Dejoie, Pamela L. Clemens, Sandy Frans, Jaime Bald, Torben Plesner, Joannes F.M. Jacobs, Niels W.C.J. van de Donk, Philippe Moreau, Jordan M. Schecter, Tahamtan Ahmadi and A. Kate Sasser.
About Cerba Research
For over 35 years, Cerba Research has been setting the industry standard for exemplary clinical trial conduct. Today, across five continents, with a focus on precision medicine, we are changing the paradigm of the central lab’s role in complex clinical research.
From protocol inception through development and to market, our passionate experts deliver the highest quality specialized and personalized laboratory and diagnostic solutions. Partner with us for the most efficient strategy to actualize your biotech and pharmaceutical products sooner and improve the lives of patients worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.