The advent of immuno-oncology and personalized medicine has led to considerable advances in cancer research, but this has not been without its challenges due to the need to work with a global patient base.
Cerba Research is at the forefront of these advances, helping to shape and progress complex trials via the company’s worldwide network of industry-leading labs, its network of scientific experts spanning five continents and the availability of Cerba HealthCare’s comprehensive patient databases.
This combination of intelligence, custom assays and on-target protocol advice is key to helping accelerate programs to market.
Enhancing research with patient- and science-driven insight
Breakthroughs in treatments for both solid tumors and onco-hematological malignancies are more prevalent and impactful than ever before. New developments, including CAR-T cell therapies, combination therapies and checkpoint inhibitors, are rapidly gaining FDA approval.
More and more immune-based therapies are entering the clinical research funnel, offering virtually unlimited options in terms of specificity and design.
Despite these advances, clinical trial challenges remain driven by issues with efficacy analysis, toxicity management and follow-up. When considering and predicting safety profiles, it is important to consider parameters such as tissue distribution, tumor addiction and surface density.
These challenges are compounded by the problem of engaging sufficient resources to accomplish these studies.
Cerba Research is a leader in oncology clinical trials with over 35 years of experience. The company’s global solutions portfolio includes a diverse array of assays suitable for use at various stages of a clinical trial.
Customers can benefit from Cerba Research’s integrated immune therapy and central laboratory services to facilitate a wide range of clinical evaluations.
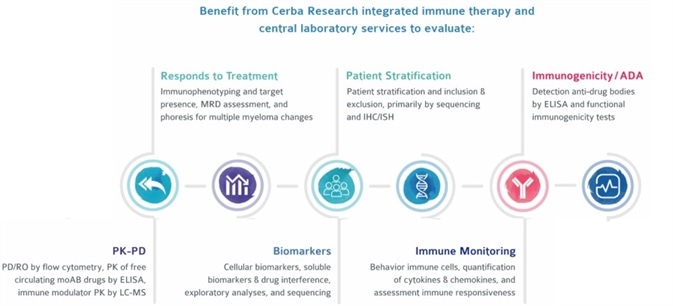
Image Credit: Cerba Research
More than half of every study that Cerba Research works with is a clinical oncology trial - from phase I through to phase IV studies and from translational and clinical research to commercialization.
Immuno-oncology is a rapidly progressing area of study, one which Cerba Research remains at the forefront of. The company offers an array of scientific expertise and state-of-the-art technologies, including:
Source: Cerba Research
| Method |
Application |
| Flow cytometry |
Immunophenotyping and pharmacokinetics |
| Immunohistochemistry |
Target presence and stratification |
| Quantitative PCR |
CAR-T cell monitoring
Vector copy number analysis (VCN) |
| PCR-based long-term safety testing |
Replication competent lentivirus (RCL) screening |
Immunoassays — ELISA and
Meso Scale Discovery (MSD) |
Toxicity (cytokine release syndrome) |
| Electrophoresis |
Multiple myeloma monitoring |
| Sequencing |
Minimal residual disease (MRD) monitoring
Patient stratification |
About Cerba Research
For over 35 years, Cerba Research has been setting the industry standard for exemplary clinical trial conduct. Today, across five continents, with a focus on precision medicine, we are changing the paradigm of the central lab’s role in complex clinical research.
From protocol inception through development and to market, our passionate experts deliver the highest quality specialized and personalized laboratory and diagnostic solutions. Partner with us for the most efficient strategy to actualize your biotech and pharmaceutical products sooner and improve the lives of patients worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.