Working alongside Cerba Research as a partner for integrated diagnostic and clinical laboratory solutions allows organizations to take complete control over the course of their complex trials.
Cerba Research’s top scientists are on hand to help assess client needs on an ongoing basis and to deliver early insights designed to optimize a protocol. These insights are beneficial at every stage, from translational research to clinical trials and commercialization.
Adjusting a unique plan to the needs of the trial
One size never fits all in today’s clinical research landscape. A successful and smooth clinical trial is built upon customer-focused project management with proactive, open communication.
Cerba Research aims to develop trusting and respectful relationships with its clients. As a midsized provider, the company is in an ideal place to offer courtesy, attention to detail and practical oversight. Cerba Research is invested in its clients’ success and its ultimate end goal of improving patients’ lives.
The advantages of partnering with Cerba Research for project management are wide-ranging and diverse.
The company’s Global Project Managers demonstrate excellent interpersonal skills and the vital ability to maintain their focus under pressure. These experts will work to understand the complexities of a client’s clinical trial, effectively managing changes throughout a project’s lifetime.
A dedicated Global Project Manager is assigned to each client organization. They work in genuine partnership with the client to determine what is important to the client and the clinical trial in question. This level of insight allows the Global Project Manager to develop customized solutions to meet the clinical trial’s needs.
Each of Cerba Research’s Global Project Managers is highly experienced in project management and working in a central lab environment. This experience allows them to lead and execute every project within budget and on time.
Each project is assigned a core project team. This team is established by the Global Project Manager and comprises a trial setup manager, a data transfer analyst and a range of subject matter experts from Cerba Research’s Science, Quality Assurance and Operations teams.
Each of these team members will be strategically aligned to support the specific needs of the clinical trial.
The company’s multi-lingual investigator site-focused team is available to support investigator sites with compliance and training to ensure successful trial delivery.
Every project will be set up and managed using a central global study database. This approach ensures both the standardization and consistency of data while being easily accessible through an online portal. Clients are afforded real-time access to their clinical trial data at any point throughout the trial.
Project teams are supported by experienced Global Portfolio Managers who are on hand to support a client’s portfolio. Their role is to ensure efficient management, consistency and standardization across the portfolio, improving overall efficiencies and ensuring streamlined and optimized portfolio and project delivery.
This work takes place inside a robust Global Project Management governance framework designed to ensure that Cerba Research is tracking and monitoring project performance on an ongoing basis.
This framework is key to ensuring service quality that exceeds client expectations.
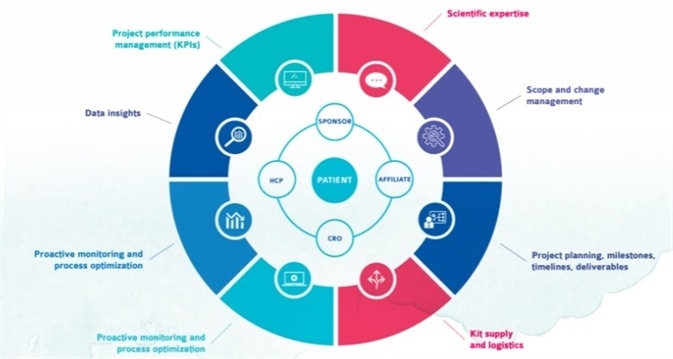
Image Credit: Cerba Research
About Cerba Research
For over 35 years, Cerba Research has been setting the industry standard for exemplary clinical trial conduct. Today, across five continents, with a focus on precision medicine, we are changing the paradigm of the central lab’s role in complex clinical research.
From protocol inception through development and to market, our passionate experts deliver the highest quality specialized and personalized laboratory and diagnostic solutions. Partner with us for the most efficient strategy to actualize your biotech and pharmaceutical products sooner and improve the lives of patients worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.