In the ever-expanding area of immuno-oncology (I/O), ensuring delivery of the appropriate treatment to the right patient at the right time demands expert, integrated clinical laboratory and diagnostic solutions executed by top scientists who will assess your needs and produce early insights that optimize your protocol.
With access to a large patient database, excellent performance across the globe and I/O experience, three main testing capabilities are imperative when choosing a lab partner for the demanding journey from translational research to commercialization.
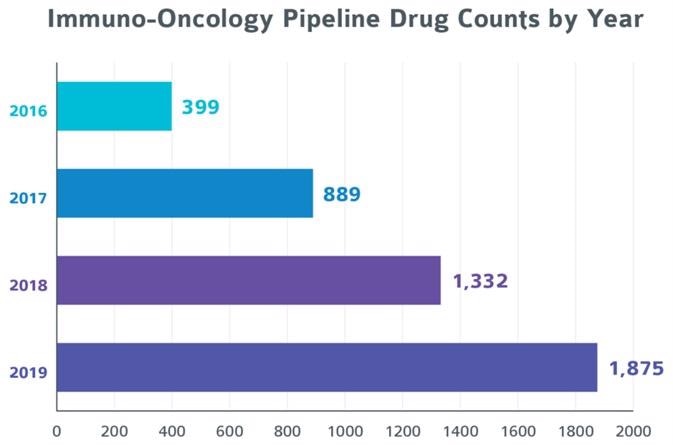
Image Source: Pharmaprojects Pharma R&D Annual Review: 2019
Biomarkers make all the difference in immunotherapeutic and personalized medicine
For a drug developer, the value of acquiring early biological insights that help determine the appropriate patients, treatments, durations and dosages, and simplify complex trials cannot be overestimated. Development and validation of Biomarkers are key to:
- Aid in prognosis
- Characterize mode of action or resistance
- Guide dose selection
- Monitor disease
- Predict drug efficacy and safety profiles
- Stratify patients/determine inclusion-exclusion
Cerba Research, which conducts half of its studies in oncology, has access to biobanked human specimens and offers a significant advantage when determining new and existing I/O-related pathways, including tumor genetics, tumor morphology, tumor protein and gene expression and tumor infiltrating lymphocytes (TILs).
The development of related biomarkers can then be conducted to filter patients into treatment groups, formulate hypotheses, gauge efficacy and increase the probability of the trial’s success.
Three types of tests support a wide variety of biomarkers
For precision medicine in immuno-oncology, a complete program that facilitates a 360˚ panorama of patient status and tumor susceptibility requires expert advice and customization for three types of testing: flow cytometry, tissue immunohistochemistry (IHC) and genetic screening (next-generation sequencing, NGS).
Flow cytometry
Flow cytometry is an influential technique that quickly identifies and quantifies thousands of cells with high specificity and sensitivity, offering a glimpse of the immune response. Aside from cell surface markers, flow cytometry can also identify intracellular antigens such as cytokines and phosphorylated signaling proteins.
This methodology facilitates functional analysis and assists in developing therapeutic strategies and predicting therapeutic responses. The synchronous use of several biomarkers produces highly complex data that is multifaceted and dimensional.
From just a single blood sample, immune profiling by flow cytometry generates a significant amount of information. The result is a fragmented breakdown, for instance, of lymphocytes and subtypes, down to T cell memory subsets and activated-versus-nonactivated markers.
Clinical researchers can use this technology to obtain knowledge relative to how patients are responding and what therapies are appropriate for patient-specific treatment plans.
These studies require scientists with high competence levels to develop and authenticate both off-the-shelf and novel biomarkers. Therefore, the lead time for assay development to validation must be taken into account.
Furthermore, for global trials, a standardized approach is crucial, including instrument standardization and assay process standardization (same SOP).
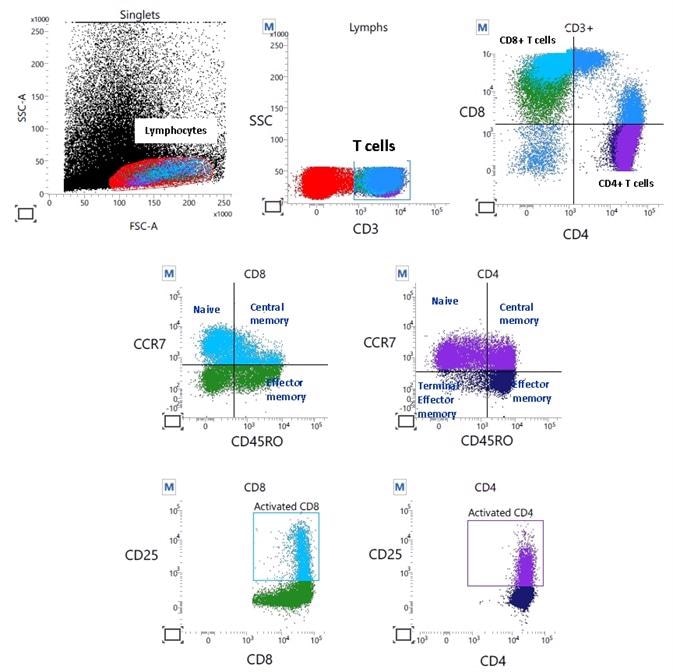
Flow cytometry: Profiling via 10-color immunophenotyping provides a detailed breakdown of lymphocyte subtypes. Image Credit: Cerba Research
Immunohistochemistry
Immunohistochemistry (IHC) is an affordable assay that profiles tissue biomarkers to enable the personalization of a patient’s therapy. It is an antibody-mediated approach that enables the detection of the target of interest in the tissue via fluorescent or chromogenic revelation for cellular localization and quantification.
This method has generally been employed to classify and diagnose tumors such as lymphomas and breast cancer. Moreover, IHC translates structural information relevant to the tumor and the tumor microenvironment, showing the localization of immune cells with regard to the tumor or other immune cell populations.
It can also show the expression of activation/deactivation biomarkers as part of immune cell profiling and oncogene assessment. Cerba Research gives sponsors an expanding number of novel I/O biomarkers, such as hard-to-develop, tailor-made IHC assays for the preclinical phase, with ensuing validation for use in clinical trials.
Multiplex IHC, which combines numerous biomarkers on a single slide/section, is a cutting-edge version that facilitates the detection of up to eight biomarkers in one extremely important tissue section. The capability to identify more biomarkers per slide is important as:
- Accurate phenotyping requires several markers
- Biopsy size limits the number of sections
- Demand for more biomarkers is growing
- Some data cannot be acquired from circulating markers, including spatial context and organization and distances between populations of cells
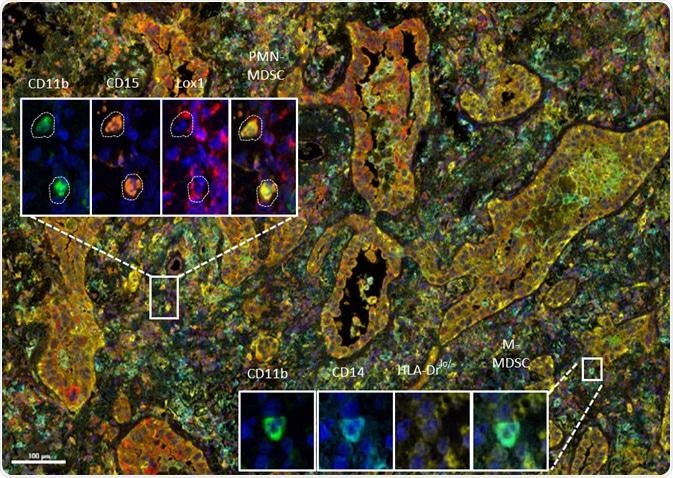
MDSC multiplex immunohistochemistry (IHC) panel developed at Cerba Research applied to non-small-cell lung cancer tissue. The high level of phenotyping allows the distinction between subtypes on a single tissue section. Shown: polymorphonuclear (PMN) myeloid-derived suppressor cells (MDSC) and mononuclear (M) MDSC. Image Credit: Cerba Research
NGS for genetic screening
Genetic screening measures variations in nucleic acid sequences related to disease susceptibility or resistance. Next generation sequencing (NGS) facilitates a wide variety of new applications and investigations in genetics, such as analysis of solid and hematologic tumor genomes as well as comprehensive analysis of the patient’s immune repertoire pre- and post-treatment, including T cell receptor (TCR) analysis.
Cerba Research’s ability to deliver high throughput, with the capability to sequence 1,000+ whole human genomes in a week — paired with one of the most extensive catalogs of clinical NGS genetic and genomic testing — helps sponsors achieve specific milestones and maintain development timelines, whether they need whole exome sequencing or customized gene panels to suit their protocol.
Applications of genetic insights to consider:
- Biomarker discovery, with detailed genomic profiling and customized assays that relate mutation to disease
- Companion diagnostics development on NGS-based or CHIP-based multiplex qPCR platforms to assess therapeutic suitability
- Cyto- and molecular-genetic diagnosis of constitutional and acquired disorders, including developmental disease, predisposition factors and clotting malfunctions
- Prospective patient stratification screening with NGS, PCR and other assays
For instance, the tumor mutational burden (TMB) is a genetic biomarker presently receiving some attention. Cancer is the consequence of a series of mutations, and cancer cell lines each have between one (1) and around 10,000 coding mutations, or .1 to 100 mutations per megabase — the tumor mutational burden.
TMB is related to antitumor response and is a good indicator of response to cancer immunotherapy drugs in some cases, such as cutaneous squamous cell carcinoma, melanoma, and certain colorectal and noncolorectal GI cancers.
The cause could be that tumor cells with increased TMB have high neoantigen loads, which leads to increased T cell reactivity and a greater antitumor T cell response. Although the benchmark for TMB analysis has been whole exome sequencing, the latest developments in NGS tumor panels have delivered consistent results.
Support for immuno-oncology trials depends on experience and commitment
With ever-growing potentialities for design and specificity, immune-based therapies are flowing into the clinical research stream. In immuno-oncology clinical trials, discerning the appropriate resources to accomplish specific goals can be a challenge.
Three primary testing methodologies are essential for immuno-oncology: multiplex immunohistochemistry (IHC) for solid tumors, flow cytometry for cells in suspension and genetic studies (NGS).
Teaming up with a high-achieving international central lab like Cerba Research for patient- and science-driven insights can help improve protocol, helping sponsors then smoothly work up to commercial scale.
Expenditures can be significantly reduced while ensuring timelines remain intact — enabling pioneering therapies to be brought to patients sooner.
With over 35 years of experience, Cerba Research is a global leader in immuno-oncology clinical trials, offering networked solutions that include a wide variety of biomarker assays and accomplished validation services.
As a partner, Cerba Research empowers customers to deliver new life-changing therapies to patients across the globe. Cerba Research helps to revolutionize the face of clinical research.
About Cerba Research
For over 35 years, Cerba Research has been setting the industry standard for exemplary clinical trial conduct. Today, across five continents, with a focus on precision medicine, we are changing the paradigm of the central lab’s role in complex clinical research.
From protocol inception through development and to market, our passionate experts deliver the highest quality specialized and personalized laboratory and diagnostic solutions. Partner with us for the most efficient strategy to actualize your biotech and pharmaceutical products sooner and improve the lives of patients worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.