There is a low probability of success for immuno-oncology (IO) research. There is a below average likelihood-of-approval rate for oncology, usually 5.3% vs. 7.9% for all indications.
The clinical trial success rate for biopharma and pharma drug developers can improve biomarkers by enhancing safety and by serving as surrogate clinical endpoints to allow for the selection of patients more likely to respond to a potential new therapeutic.
Statistically, the approval rate increases five-fold when biomarkers are used for patient stratification.
It is always a quandary trying to create a comprehensive clinical development plan to increase the effectiveness of a clinical trial through the integration of biomarkers and the process of choosing from the lengthy number of candidates.
Looking at genomic biomarkers or systemic protein in bodily fluids or circulating tumor or immune cells is the less-invasive approach.
However, critical information from tissue biopsies that cannot be observed at the systemic level by pathologists can be obtained in oncology.
Pathologists can observe not only the presence of an antitumoral response but also information on its presence and actual engagement at the tumor level using classic techniques such as in situ hybridization (ISH) and immunohistochemistry (IHC).
This means that biopsies are necessary in clinical trials when monitoring the effectiveness of anti-blockade treatments.
Histology (IHC) helps us understand biomarker mechanisms in the tumor environment
Immunohistochemistry technology, historically and remains today, the gold standard of clinical pathology. It is a readily available, cost-effective and simple (Fig. 1) method for profiling biomarkers for the purpose of individualizing a patient’s therapy.
How IHC works
Very different information is offered by IHC as compared with other types of biomarker approaches. For example, flow cytometry evaluates individual cells in suspension with no fixed relationship. Genetic screening like NGS examines how the sample corresponds to a homogenization of the tissue.
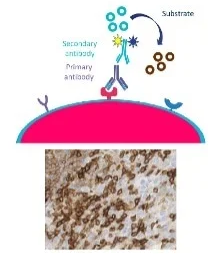
Figure 1. Antibody-mediated fluorescent or chromogenic stains mark the presence and location in the solid tumor. Image Credit: Cerba Research
Using IHC, the context of the target within the tumor micro-environment can be appreciated. A key advantage of IHC is learning where types of cells are located or where the target is in relation to other structures, meaning there is a possibility for spatial analysis.
IHC provides a greater understanding of the following:
- Immune response status at tumor site
- Spatial relationships between cells of immune, stroma and tumor origin
- Structure of tumor
- Microenvironment of tumor
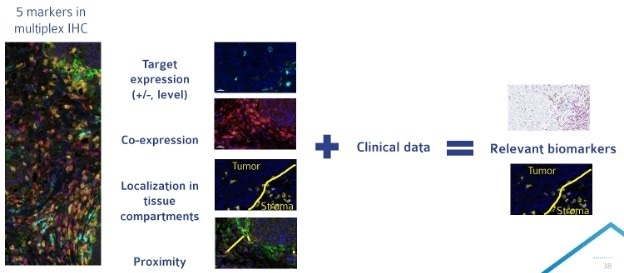
Figure 2. Multiplex IHC allows for the analysis of a large number of parameters on a single tissue section. Compiling multiplex IHC results with clinical data can allow for the identification of a more targeted biomarker approach for late phase clinical trials. Image Credit: Cerba Research
The value of multiplex IHC
The complexity of immuno-oncology has made it apparent that a single biomarker may not be sufficient, leading to a need for multiplexing techniques in both clinical and preclinical studies.
Multiplex IHC broadens mechanistic investigations by allowing for the detection of up to 8 biomarkers on a single tissue section.
Biomarker research can harness the power of multiplex IHC by allowing for a myriad of analyses, including target localization (e.g., Ligand in relation to a receptor, protein expression on specific cell types) and in-depth immune cell phenotype analysis (e.g., regulatory T cell — CD3+/CD4+/CD25+/FoxP3+).
Multiplex IHC is especially useful in early and retrospective clinical studies as well as for preclinical studies. Early investigations can be more flexible for studying a panel of candidate biomarkers that could (Fig. 2) accomplish the following:
- Examine how different cell types interact: are they possibly communicating? Are they within proximity of one another?
- Profile immune cells and understand the different subtypes within the tissue (such as CD8 T cells). An example of this would be studying if immune cells are on the periphery (cold) or within the tumor (hot).
- Examine the mechanism — the biology of interactions in the tumor microenvironment between the tumor, the infiltrating immune cells and the stromal cells that are supporting the tumor.
- Find a specific drug target of interest, examine its level of expression, and determine its localization. It is then possible to ask if it is expressed within the tumor or the healthy tissue and if it is expressed on a particular cell type.
The combination of clinical and multiplex data can then be used to identify a more focused biomarker approach that is more applicable in later phase clinical trials.
Cerba Research has one of the first multi-spectral imaging systems in Europe, as well as years of multiplex IHC experience, and has additionally developed off-the-shelf panels with several common immuno-oncology targets.
The company has the technology and the expertise to help scientists understand the neighborhoods of tissue sections, and their work is always with respect for the patients behind the samples.
Fit-for-purpose IHC assays must be validated for the intended use
A fit-for-purpose assay can be generated once the basics have been determined: what type of analysis is relevant, the intended use (for example, mechanism or proof of principle), what tissues are being studied, which biomarkers are relevant.
The successful integration of biomarkers into a clinical trial means that it is crucial to have a robust biomarker validation process. There is a requirement for collaboration between clinicians and the specialty lab at each stage of clinical and preclinical development with special attention to precision, sensitivity, specificity and protocol optimization.
The IHC expert scientific team at Cerba Research is flexible in their approach and delivery for the provision of cost-effective and timely solutions to meet commercial and clinical objectives.
Solutions from Cerba Research include:
- Support technologies, including antibody target screening, cell culture, Nanostring and mass cytometry
- IHC expert guidance
- Access to numerous indications in our tissue biobank to facilitate target detection in multiple disease areas
- Custom IHC assay and validation development for clinical and preclinical studies
- Available protocol catalog
Shape your immuno-oncology research with IHC
A unique tool in immunotherapy and cancer research is the detection of specific antigens in tissue sections by IHC. Far more detailed insights can be provided this way than through standard histopathology.
Tissue biomarkers can yield crucial insights for mechanistic evaluation, patient stratification and diagnosis with special techniques such as multiplex IHC and ISH. Proper validation improves the chances for success in I/O clinical trials, provides confidence in the results and is required for regulatory acceptance.
About Cerba Research
For over 35 years, Cerba Research has been setting the industry standard for exemplary clinical trial conduct. Today, across five continents, with a focus on precision medicine, we are changing the paradigm of the central lab’s role in complex clinical research.
From protocol inception through development and to market, our passionate experts deliver the highest quality specialized and personalized laboratory and diagnostic solutions. Partner with us for the most efficient strategy to actualize your biotech and pharmaceutical products sooner and improve the lives of patients worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.