In the fields of personalized and precision medicine, diagnostics and pharmaceuticals are quickly merging together. Cerba Research supports clients and their patients through the accomplished development of companion diagnostics.
A companion diagnostic test is an in vitro device that offers relevant information concerning the safety and efficacy of a particular therapeutic product. It can:
- Determine patients most likely to benefit
- Determine patients likely to be at a greater risk of severe side effects
- Monitor treatment responses, allowing adjustments for enhanced safety or effectiveness
Amassing the additional biomarker and genomic data required to enhance and accelerate the decision-making process in oncology and, specifically, immuno-oncology expedites drug development. It may also refine approval rates: Drugs filed with companion diagnostics have a 30% rate of FDA approval, in contrast to 8% for those without.
Partner with Cerba Research to develop companion diagnostics
Capitalize on Cerba Research’s experience in developing IVD assays for use as CDx.
- Cerba Research is focused on IO, hematological cancers and lung cancer
- 60% of Cerba Research trials are in oncology
- Improved and more integrated access to both US and European markets is a crucial requirement for oncology trials as they become more global and increase in both cost and complexity
Clients can streamline their strategy and co-develop a companion diagnostic together with a therapeutic agent early on.
Execute regulatory-related best practices for the greatest chance of rapid approval for the combined therapy and companion diagnostic.
- Conform to the strict regulations for both therapies and devices
- Therapeutic Product: Investigational New Drug, 21 CFR 312
- IVD: Investigational Device Exemption (IDE) Regulation, 21 CFR 812
- Optimize clinical trial development to include investigation of IVD companion diagnostic alongside the therapy
- Guarantee appropriate validation of companion diagnostic before trial starts to avoid any later issues
- CDx must be sufficiently analytically robust, specifically around the test’s clinical decision point(s)
- Only use tests with “market-ready” performance in key/Phase III trials
- Acquire expert assistance to simplify the submission process for the therapeutic product and CDx
Cerba Research’s combined expertise in biomarker discovery and companion diagnostics development facilitates the precision oncology efforts of its global partners. Join forces with Cerba Research to advance development and promote regulatory and commercial success.
Pharmaceutical development stages
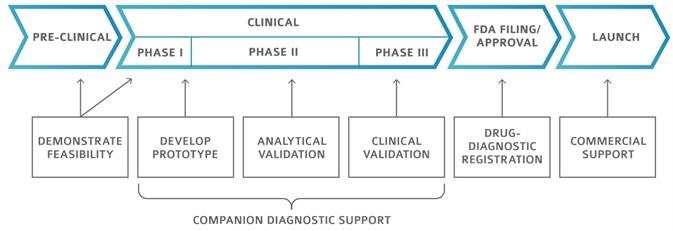
Image Credit: Cerba Research
Cerba Research offers a complete range of companion diagnostic support, from prototype development to clinical validation. Cerba helps to revolutionize the face of clinical development.
As a partner, Cerba Research grants clients the power to offer new, life-changing therapies to patients around the world.
About Cerba Research
For over 35 years, Cerba Research has been setting the industry standard for exemplary clinical trial conduct. Today, across five continents, with a focus on precision medicine, we are changing the paradigm of the central lab’s role in complex clinical research.
From protocol inception through development and to market, our passionate experts deliver the highest quality specialized and personalized laboratory and diagnostic solutions. Partner with us for the most efficient strategy to actualize your biotech and pharmaceutical products sooner and improve the lives of patients worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.