The illicit drugs fentanyl and methamphetamine are of key interest to law enforcement.
When utilized in direct (point-and-shoot) mode, the Thermo Scientific™ TruNarc™ analyzer can routinely differentiate and identify these two drugs. However, employing the instrument with the TruNarc H-Kit method will produce the identification “Fentanyl Compound and/or Methamphetamine” due to the near-identical H-Kit spectra produced by the two drugs, as shown in Figure 1.
Previously, scanning fentanyl or methamphetamine in direct mode occasionally produced a “Fentanyl Compound and/or Methamphetamine” identification, requiring further steps to guarantee accurate results.
To avoid this outcome, the library spectra for H-Kit and direct scans have been completely separated in software version 1.9.0, enhancing the performance of the instrument. As a result, the operator is now required to select either direct scan or H-Kit prior to testing.

Image Credit: Thermo Fisher Scientific – Portable and Handheld Raman Spectroscopy
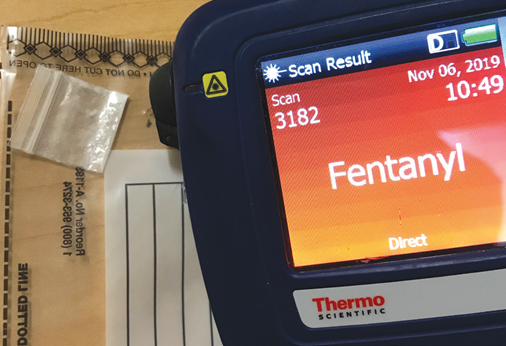
Image Credit: Thermo Fisher Scientific – Portable and Handheld Raman Spectroscopy
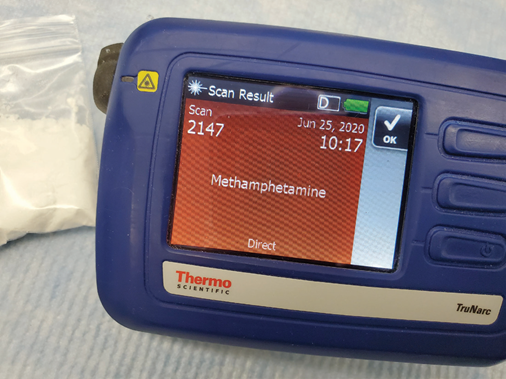
Image Credit: Thermo Fisher Scientific – Portable and Handheld Raman Spectroscopy
Additionally, this software version (and future iterations) allows the operator to switch off the H-Kit scanning capability, eliminating the possibility of inconclusive identification.
Liquid methamphetamine
For liquid methamphetamine, operators are encouraged to allow the liquid to completely evaporate before performing a direct scan. Evaporation can be achieved by placing the liquid on the surface of paper or glass and waiting for crystals to form.
Scanning these crystals in direct mode results in the correct identification of methamphetamine.
General recommendations
It is recommended that a direct scan is always attempted initially and that scans are performed at multiple points on the sample to account for inconsistent composition.
In cases where a direct scan fails to identify the substance, an H-Kit scan should be attempted. In all instances, results should be checked against a secondary analysis technique, particularly in cases where the instrument analysis results are inconsistent with known information about the sample and other evidence in the case.
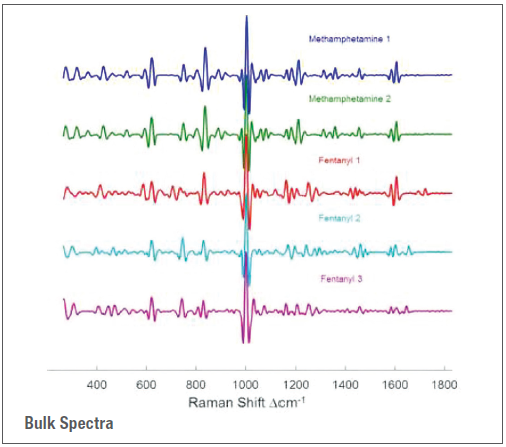
Image Credit: Thermo Fisher Scientific – Portable and Handheld Raman Spectroscopy
About Thermo Fisher Scientific – Portable and Handheld Raman Spectroscopy
Thermo Fisher Scientific offers innovative solutions that help our customers solve complex analytical challenges, accelerate life sciences manufacturing, deliver medicines to market, and increase laboratory productivity. Our Thermo Scientific portable and handheld process Raman analyzers enable accurate, real-time results for process monitoring.
The Thermo Scientific™ MarqMetrix™ All-In-One Process Raman Analyzer is an all-in-one system purpose-built for rapid deployment, ease of use, and scalability in markets where time-to-results is critical. The MarqMetrix All-In-One Process Raman Analyzer is designed for:
- Analysis without sample preparation, delivering Raman spectral results in real-time
- Easy setup and deployment by non–Raman spectroscopists
- Non-destructive workflows to protect precious samples
- Non-invasive handling to minimize contamination of samples
- Small footprint for convenient deployment
- Factory calibration for hardware stability and portability
Our Thermo Scientific TruScan™ Handheld Raman Analyzers includes state-of-the-art optics paired with a patented multivariate residual analysis that offers an effective chemometric solution for material identification, with two spectral pre-processing options. The non-destructive point-and-shoot sampling principle facilitates rapid verification of a broad range of chemical compounds, including cellulose-based products.
The TruScan™ G3 Handheld Raman Analyzer continues the evolution of raw material ID testing for pharmaceutical and biotechnology manufacturing quality control processes with:
- Incoming raw material identification (RMID)
- Dispensing of materials during API manufacture
- Finished product inspection
- Identification of falsified (counterfeit) medicines
- Enables non-technical operators to rapidly perform raw material ID testing without the need of a lab
The TruScan™ RM Handheld Raman Analyzer takes pharmaceutical manufacturing QA/QC to the next level with:
- Enhanced 21 CFR Part 11 and cGMP compliance with biometric login, complex password options, and full audit trail features
- Non-contact analysis through plastic bags, glass containers, blister packs and clear gel caps
- Intuitive workflow adapted to production environment through PDF batch reports and ease of data input using barcode scanners
- Easy fleet management feature that enables cloning of instruments and identification methods
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.