Magnetic resonance imaging (MRI) is a popular imaging modality in preclinical research because it delivers multiparametric information on tissues and organs in a noninvasive manner. The small dimensions of the animal organism necessitate excellent spatial resolution, which places a high demand on the signal-to-noise ratio.
MRI systems use magnets that operate at high and ultra-high fields, specific radiofrequency coils, and scanner electronics to produce images with high SNR.
However, thermal object and receiver noise can impair the quality of MR images, which is determined by specific hardware settings, the kind of tissue scanned, and sequence parameters.
Noisy images can occur when: (i) small voxel volumes are used to spatially resolve specific structures, (ii) averaging is not desirable or practical, (iii) methods requiring signal attenuation for contrast generation, such as diffusion MRI and relaxometry, or (iv) parallel imaging techniques are used.1, 4
Images with high noise levels may impede visual interpretation and result in insufficient sensitivity to detect minor signal changes, as seen in functional MR investigations.
Processing procedures such as registration or quantitative MRI, such as tensor estimation in diffusion tensor imaging, may be more difficult.3
Denoising techniques to improve qualitative and quantitative assessments of noisy images are being used in the MRI area.5
Smart noise reduction
With ParaVision 360 V3.6, Bruker launched Smart Noise Reduction, a unique image reconstruction technique for reducing and removing noise from MR images.
The Smart Noise Reduction image reconstruction approach relies on residual convolutional neural networks that have learned the noise structure and can remove it from the original data.
The networks were trained only by supervised learning, with no generative approaches. A data consistency factor allows for variable denoising levels, preventing oversmoothing while maintaining visual contrast.
This data consistency and an iterative denoising (pre-denoising) approach can significantly improve the quality of the final images.
Smart Noise Reduction uses three neural networks with different structures and sizes for the denoising method. While smaller networks (network Quick) have faster processing times, larger networks (networks Strong and Large) have lower noise needs.
Fig 1 depicts exemplary denoising results for these three networks. A denoised 3D dataset was used as a reference to demonstrate the effect of denoising with different networks.
Two generated noise levels (standard deviation 0.05 and 0.1) were added to the reference image, which was then fed into the three denoising networks (Quick, Strong, and Large). All datasets were denoised with 50% pre-denoising and a denoising intensity of 100%.
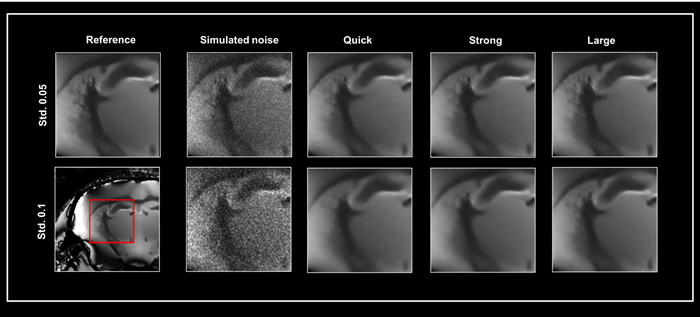
Figure 1. Smart Noise Reduction using different neuronal networks. A denoised 3D dataset was taken as ground truth (Reference) to which two varying levels of simulated noise were added, i.e. standard deviation (Std.) 0.05 and 0.1, respectively. Noisy data were subsequently reconstructed with the Quick, Strong, and Large networks. All data were denoised with a 100 % denoising level and 50 % pre-denoising. The area shown in the red box was selected for the similarity metrics in Table 1. Image Credit: Bruker BioSpin Group
Performance evaluation
The major problem in denoising structural MR images is reducing the noise while preserving the features, edges, and small structures that may be important for image interpretation and analysis.
Furthermore, the process should not produce visual artifacts or include features that do not exist in the topic.
To assess the quality of the rebuilt images presented in Fig 1. The Peak Signal to Noise Ratio (PSNR) and Structural SIMilarity (SSIM) indices between the reference image and the data reconstructed using the three different networks were computed (Table 1). The chosen area for analysis is highlighted in Fig 1.
While big networks provide the highest performance, quick reconstruction with Quick and Strong networks produces high-quality images.
Table 1. Quantitative comparison of performance of three neuronal networks for Smart Noise Reduction. For the SSIM index 0 indicates no similarity and 1 indicates perfect similarity. Bold indicates the best performing network. Source: Bruker BioSpin Group
| Noise |
Metric |
Quick |
Strong |
Large |
| Std. 0.05 |
PSNR |
37.272 |
38.592 |
39.152 |
| SSIM |
0.9439 |
0.9657 |
0.9711 |
| Std. 0.1 |
PSNR |
34.239 |
35.380 |
35.939 |
| SSIM |
0.9332 |
0.9483 |
0.9531 |
In addition to network options, the denoising level can be selected. Fig 2A shows the effect of applying various levels of denoising to image reconstruction.
Mouse brain data was obtained ex vivo, and the original data was rebuilt without and with increasing denoising levels (70-100%). Increasing the level of denoising produces images with gradually less noise. Importantly, no artifacts were introduced during the procedure.
Computing the difference images between the source and 70% denoised images revealed that only noise was selectively eliminated. If the original signal at the image's edges is reduced due to bandwidth selection, high denoising levels (90 and 100%) can result in a hazy look of edges.
The appropriate denoising level, which balances efficient noise removal with edge blurring, must be determined for each data set or, at the very least, each application protocol.
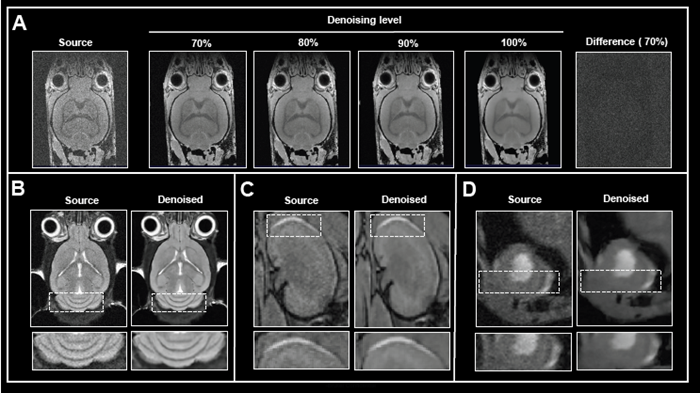
Figure 2. The effect of image denoising on image quality. A) Applying increasing levels of denoising. Shown are axial images of a 3D T1-weighted FLASH ex vivo data of a fixed mouse head acquired at 9.4 Tesla without (Source) and different levels (70-100 %) of denoising. A difference image between the source and 70 % denoised images was computed. B)-D) Comparison of in vivo images reconstructed with no denoising and with 70 % denoising. B) Coronal T2-weighted TurboRARE images of a mouse brain acquired at 3 Tesla. C) Coronal 3D FISP images of mouse kidney acquired at 7 Tesla. D) Short axis view of a mouse heart acquired with a flow-compensated triggered FLASH sequence at 9.4 Tesla. For different data, either no denoising (Source) or denoising using individual networks and a pre-denoising of 50 % was applied (Denoised). Image Credit: Bruker BioSpin Group
The efficacy of Smart Noise Reduction was illustrated using in vivo mouse data from various organs collected at various magnetic field strengths (Fig 2B-D).
Compared to reference images of the brain, kidney, and heart, reconstruction with 70% denoising resulted in images with substantially less noise and a better look of anatomical details and edges.
To test the efficacy of the denoising algorithm with images of varying levels of tissue contrast, ex vivo data of a fixed mouse brain was obtained with a nominal voxel size of 55 x 55 x 800 µm3 at 3 Tesla, either with no averaging, 4, or 15 averages (Fig 3).
The contrast-to-noise ratio for the corpus callosum and cortex is 2.47±0.45, 4.59±0.01, and 12.03±0.81, respectively. Images were reconstructed without 70% and 100% denoising.
Given the relatively high resolution, the image without averaging has low tissue contrast and noise, which can be reduced by utilizing averaging during acquisition or denoising during reconstruction.
Comparing denoised images taken with varying average numbers demonstrates that denoising might magnify false contrast in circumstances of low tissue contrast, i.e. no averaging (Fig 3, arrow).
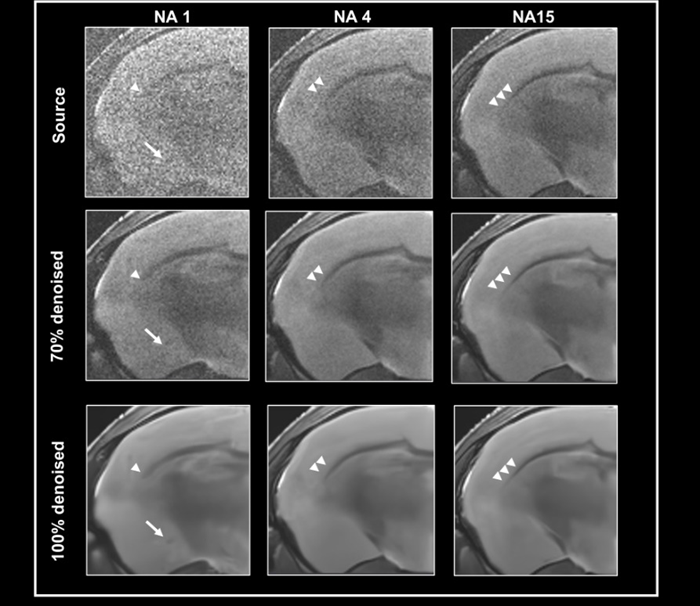
Figure 3. Denoising images with low tissue contrast. A) Axial T2-weighted TurboRARE images of a fixed mouse head were acquired at 3 Tesla with 1, 4 and 15 number of averages (NA), respectively. Images have a nominal voxel resolution of 55 x 55 x 800 μm3. Image reconstruction was performed without (Source) and with 70 % and 100 % denoising. Each denoised image was reconstructed with a network Strong and a pre-denoising of 50 % was applied. The arrows point to a structure in the NA1 images, that is faintly visible in the image that has not been denoised and that becomes more apparent with increasing denoising. The arrowheads point to the corpus callosum which becomes more visible. Resolution of fine structure requires high tissue contrast with averaging. Image Credit: Bruker BioSpin Group
This structure, which is slightly visible in the noisy image without denoising but is difficult to differentiate from the surrounding tissue, is improved by raising the level of denoising.
A comparison of images with varying amounts of averaging shows that denoising makes the corpus callosum more visible. To resolve the fine structure of the corpus callosum's extent, strong tissue contrast and averaging are required. If this is the case, denoising improves visualization.
Faster imaging
MRI data acquisition is an inherently slow operation, with acquisition times increasing with higher spatial resolution, huge volumetric coverage, and/or the collection of many contrast images or quantitative data sets. Obtaining high SNR data in a reasonable acquisition time is quite desirable.
This article shows the application of Smart Noise Reduction to denoise rapid structural brain scans. Fig 4 depicts instances of ex vivo brain scans with various contrasts and orientations collected in less than five minutes. The data was collected from sensors operating at 3, 7, and 9.4 Tesla.
To accomplish the desired short acquisition time, conventional procedures produced for each system were modified to remove averaging (Fig 4A-C). This resulted in 7-15 faster acquisition times than the original protocols.
With the specified resolution, however, no averaging produced noisy images. Reconstructing the obtained data with the denoising algorithm effectively reduced noise from images and produced high-quality results.
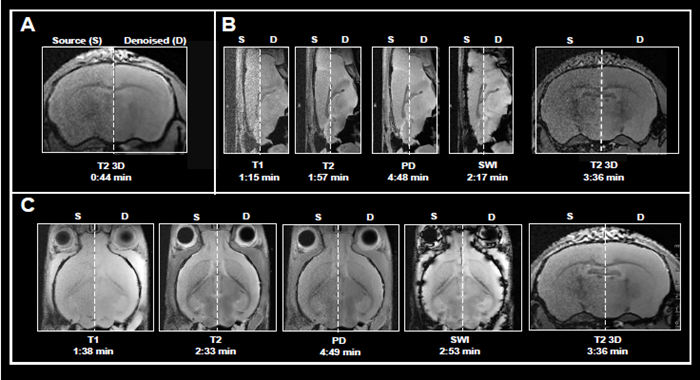
Figure 4. Examples of fast brain scans. Ex vivo data of a fixed mouse head acquired with different image contrasts, geometries and orientations. Data were acquired at A) 3 Tesla, B) 7 Tesla, and C) 9.4 Tesla. Images were reconstructed with no denoising (Source) and with a network Strong and applying 50 % pre-denoising and a denoising level of 70 % (Denoised). Image Credit: Bruker BioSpin Group
Several ways to speed up MRI acquisitions by utilizing temporal or spatial redundancy of images have been proposed in the literature.2, 6 Parallel imaging techniques provide structured noise in the reconstructed image because of the parallel reconstruction algorithm's reduced data sampling and noise amplification.1
Ex vivo brain data were collected using either partial Fourier or the multi-coil generalized auto-calibrating partial parallel acquisition (GRAPPA) method (Fig 5). The data obtained without acceleration served as a reference. Acceleration resulted in up to a threefold reduction in acquisition times compared to the protocol without averaging.
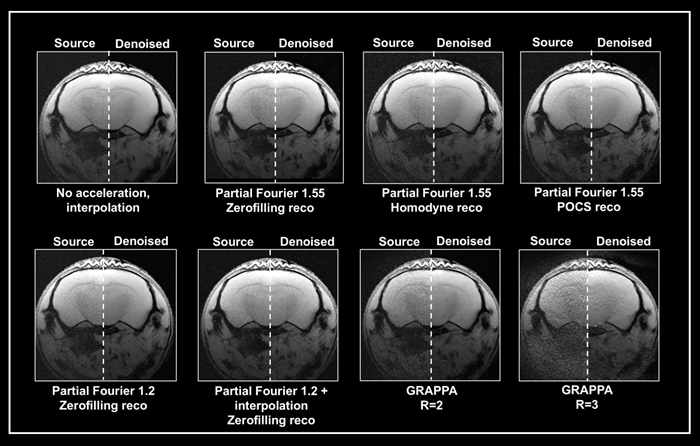
Figure 5. Denoising of accelerated brain data. Ex vivo T1-weighted FLASH data of a fixed mouse head were acquired at 9.4 Tesla. Data were either acquired with no acceleration, with partial Fourier (in Read), partial Fourier and interpolation (1.35 in Read and Phase), or with multi-coil parallel imaging technique GRAPPA. For GRAPPA, an acceleration factor (R) of 2 and 3 was chosen, respectively. Partial Fourier images were reconstructed either with zerofilling, homodyne, or POCS, as indicated. All images are shown with no denoising (Source) and after denoising (Denoised) with a Strong network, applying a pre-denoising level of 50 % and denoising level of 70 %. Image Credit: Bruker BioSpin Group
A denoising level of 70% resulted in greater residual noise in data acquired with a partial Fourier 1.55 than in data acquired without acceleration. The effect was largest in homodyne-reconstructed data and weaker in zero-filled data.
Denoising of these accelerated datasets resulted in image blurring, which was largest in homodyne reconstructed images and lowest in zerofilling reconstructions.
When images were acquired using partial Fourier 1.2, they had less residual noise and appeared normal when denoised with 70%. Denoising, on the other hand, was ineffective when combining a partial Fourier with interpolation (1.35 in Read and Phase) and the source and denoised images had comparable appearances.
Grappa acceleration produced noisier images compared to partial Fourier capture. Reconstructing the collected data using the denoising method successfully reduced noise from the GRAPPA image acquired with an acceleration factor of 2.
However, in the image captured with an acceleration factor of 3, the noise is not totally erased. Moreover, reconstruction artifacts and loss of tissue contrast unrelated to the denoising reconstruction are visible.
This suggests that the method may be unable to deal with data with noise characteristics that differ from those used to train the networks. However, the outcome is determined by the parameters used for acquisition and reconstruction.
Denoising may improve images with low SNR by using quicker imaging techniques. The overall decrease in acquisition time that can be accomplished by adapting imaging procedures can dramatically reduce animal exposure time in the instrument, allowing for method refining.
This also enables the acquisition of extra read-outs during an MRI examination and increases research throughput.
Boosting the resolution of images per unit time
High-resolution MRI offers precise structures in tissue and organs, making it useful for detecting anomalies such as lesions or cancers. To compensate for the inherent low SNR in high-resolution structural MRI studies, extended image acquisition durations are required.
This article shows how Smart Noise Reduction may be utilized to reduce acquisition times while retaining image quality.
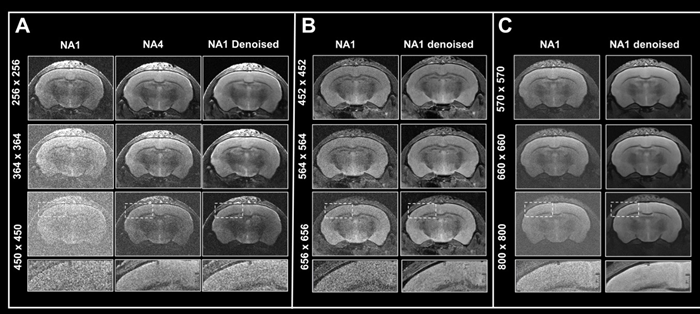
Figure 6. Boosting resolution. Ex vivo T2-weighted TurboRARE data of a fixed mouse head acquired at A) 3 Tesla, B) 7 Tesla, and C) 9.4 Tesla. All images were acquired with 0.8 mm (A) and 0.7 mm (B, C) slice thickness and with a field-of-view of 20 x 20 mm2 and the given matrix size using either no averaging or acquiring 4 averages. Images were reconstructed with no denoising (NA1 and NA4), or after denoising (NA1 Denoised) using a network Large, applying a pre-denoising level of 50 % and denoising level of 70 %. Image Credit: Bruker BioSpin Group
In Fig 6, axial T2-weighted TurboRARE images of a fixed mouse head taken at various field strengths are compared. Images were obtained with a fixed field-of-view of 20 x 20 mm2, slices that were 0.8 mm (3 Tesla) and 0.7 mm (7 and 9.4 Tesla) thick, and changing matrix sizes to produce images with various spatial resolution.
For images acquired at 3, 7, and 9.4 Tesla, the matrix sizes were raised by up to 1.8, 2.6, and 3.1 (compared to the reference protocols' matrix size of 256). These resolution upgrades cause a reduction in SNR for each image.
The loss in SNR can be accounted for by averaging, therefore extra data was collected using four averages.
A comparison of non-denoised averaged images and single averaged images that were denoised demonstrates that denoising reduces increased image noise at higher resolutions and produces images of comparable quality to those generated through averaging.
The advantages in image quality from denoising are greater with data recorded at 3 Tesla, when increasing resolution provides images that are more difficult to interpret, as opposed to data acquired at 7 and 9.4 Tesla, where SNR is naturally higher.
Nonetheless, images taken at 7 and 9.4 Tesla benefit significantly from denoising during reconstruction since the intrinsic higher SNR of these images may be used to pick smaller voxel sizes.
This shows that denoising allows for better resolutions within a given unit time by reducing the requirement for averaging. This can also be useful when averaging is neither desirable nor practical.
Conclusion
Smart Noise Reduction efficiently eliminates noise from structural images, improving the image quality of acquired data. With denoising levels and network settings, the user can tailor denoising results to their own applications and needs.
Noisy images are enhanced when SNR is limited, such as images taken at low field strengths, when ideal coils are inaccessible, or when averaging is not feasible or desirable. When SNR is sufficient, denoising can be employed to increase resolution per unit time or to speed up acquisitions.
Abbreviations
- CNR = contrast-to-noise ratio
- FISP = fast imaging with steady-state precession
- FLASH = fast low-angle shot
- GRAPPA = generalized autocalibrating partial parallel acquisition
- MRI = magnetic resonance imaging
- NA = number of averages
- PSNR = peak signal-to-noise ratio
- RARE = rapid acquisition with relaxation enhancement
- SNR = signal-to-noise ratio
- SSIM = structural similarity
- SWI = susceptibility weighted imaging
References
- Aja-Fernández, S., Vegas-Sánchez-Ferrero, G. and Tristán-Vega, A. (2014). Noise estimation in parallel MRI: GRAPPA and SENSE. Magnetic Resonance Imaging, 32(3), pp.281–290. https://doi.org/10.1016/j.mri.2013.12.001.
- Griswold, M.A., et al. (2002). Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Medicine, [online] 47(6), pp.1202–1210. https://doi.org/10.1002/mrm.10171.
- Huang, H., et al. (2004). Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. 52(3), pp.559–565. https://doi.org/10.1002/mrm.20147.
- Macovski, A. (1996). Noise in MRI. Magnetic Resonance in Medicine, 36(3), pp.494–497. https://doi.org/10.1002/mrm.1910360327.
- Mishro, P.K., et al. (2022). A Survey on State-of-the-Art Denoising Techniques for Brain Magnetic Resonance Images. IEEE Reviews in Biomedical Engineering, 15, pp.184–199. https://doi.org/10.1109/rbme.2021.3055556.
- Pruessmann, K.P. et al. (1999). SENSE: Sensitivity encoding for fast MRI. Magnetic Resonance in Medicine, 42(5), pp. 952–962. https://doi.org/10.1002/(sici)1522-2594(199911)42:5.
About Bruker BioSpin Group
The Bruker BioSpin Group designs, manufactures, and distributes advanced scientific instruments based on magnetic resonance and preclinical imaging technologies. These include our industry-leading NMR and EPR spectrometers, as well as imaging systems utilizing MRI, PET, SPECT, CT, Optical and MPI modalities. The Group also offers integrated software solutions and automation tools to support digital transformation across research and quality control environments.
Bruker BioSpin’s customers in academic, government, industrial, and pharmaceutical sectors rely on these technologies to gain detailed insights into molecular structure, dynamics, and interactions. Our solutions play a key role in structural biology, drug discovery, disease research, metabolomics, and advanced materials analysis. Recent investments in lab automation, optical imaging, and contract research services further strengthen our ability to support evolving customer needs and enable scientific innovation.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.