Interferon-gamma (IFN-γ/IFNG) is a dimerized soluble cytokine and the sole member of the type II interferon class. Originally referred to as macrophage-activating factor, IFN-γ is now recognized as part of a broader family of proteins involved in immune regulation. It has been explored for various clinical applications due to its central role in immune responses.
IFN-γ signals through the Janus Activated Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathway, playing a key role in both innate and adaptive immunity. It enhances antiviral and antitumor responses by upregulating genes involved in cell cycle regulation, apoptosis, and antigen processing/presentation. However, rodent and human trophoblast cells exhibit reduced sensitivity to IFN-γ, which contributes to their resistance to IFN-γ-mediated activation of major histocompatibility complex (MHC) class II antigen expression.
Features
- Designed under ISO 9001:2015 and ISO 13485:2016 standards
- Manufactured and QC tested in a GMP-compliant facility
- Made with animal-free materials
- Free from beta-lactam materials
- Ensures batch-to-batch consistency
- Subjected to stringent quality control tests
Source
GMP Human IFN-Gamma Protein (Cat. No. GMP-IFGH24) is expressed in human 293 cells (HEK293) and consists of amino acids Gln24–Gln166 (Accession # P01579).
Predicted N-terminus: Gln 24
Molecular characterization

Image Credit: ACROBiosystems
This protein carries no tag.
It has a calculated molecular weight of 16.8 kDa but migrates at approximately 15 kDa, 19 kDa, and 24 kDa ±3 kDa under reducing (R) conditions in SDS-PAGE due to glycosylation.
Endotoxin
Using the LAL method, less than 10 EU/mg.
Host cell protein
ELISA-tested protein: less than 0.5 ng/µg.
Host cell DNA
qPCR-tested protein at less than 0.02 ng/μg.
Sterility
The membrane filtration method outlined in CP<1101>, USP<71>, and Eur. Ph. 2.6.1 was used to conduct the sterility test.
Mycoplasma
Negative.
Purity
>95 %, according to SDS-PAGE.
Formulation
Lyophilized in PBS at pH 7.4 with protectants from a 0.22 μm filtered solution.
Shipping
This product is supplied and shipped with blue ice.
Storage
Upon receipt, store immediately at -20°C or lower for long-term storage.
Avoid repeated freeze-thaw cycles.
This product remains stable under the following conditions:
- -70 °C for 12 months under sterile conditions after reconstitution
- -20 °C to -70 °C for 5 years in lyophilized state
ACRO quality management system
- QMS(ISO, GMP)
- Quality Advantages
- Quality Control Process
QC and assay protocol
SDS-PAGE
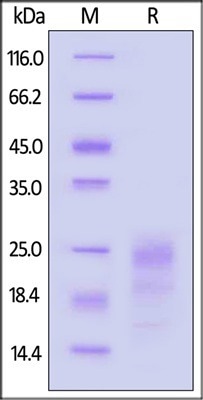
Image Credit: ACROBiosystems
GMP Human IFN-Gamma Protein was analyzed by SDS-PAGE under reducing (R) conditions. The gel was stained with Coomassie Blue, confirming a purity of greater than 95 %.
Bioactivity-Bioactivity CELL BASE
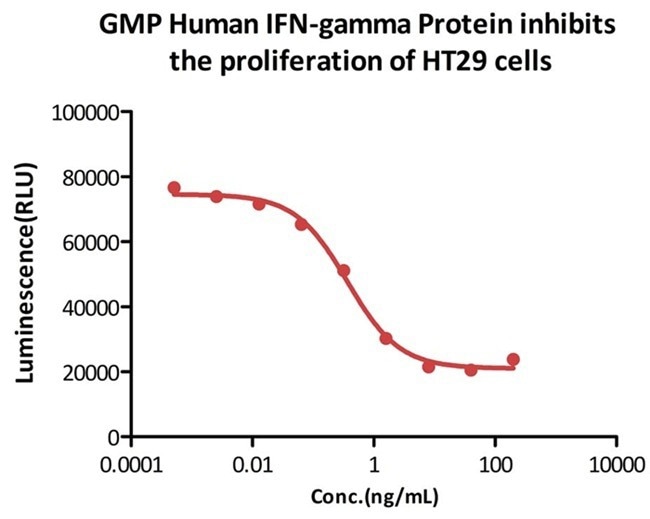
Image Credit: ACROBiosystems
GMP Human IFN-Gamma Protein (Cat. No. GMP-IFGH24) inhibits the proliferation of HT-29 cells. Its specific activity is >2.00 × 107 IU/mg, calibrated against the human interferon gamma standard (NIBSC code: 87/586) (QC tested).
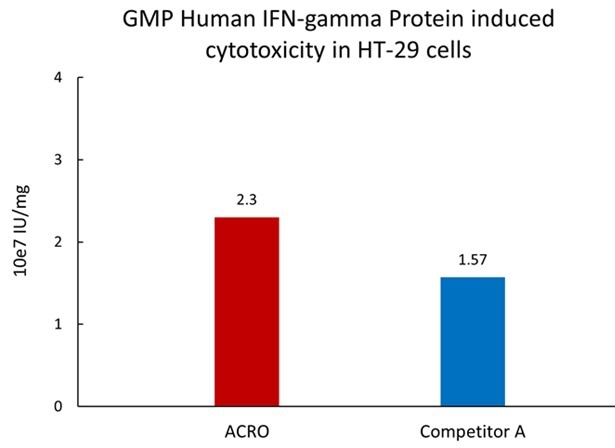
Image Credit: ACROBiosystems
Compared to competing products, GMP Human IFN-gamma Protein (Cat. No. GMP-IFGH24) exhibited a higher activity level.
Bioactivity-Stability
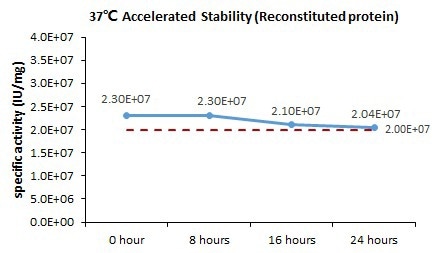
Image Credit: ACROBiosystems
GMP Human IFN-gamma Protein (Cat. No. GMP-IFGH24) is stable at 37 °C for 24 hours, according to the cell-based assay.
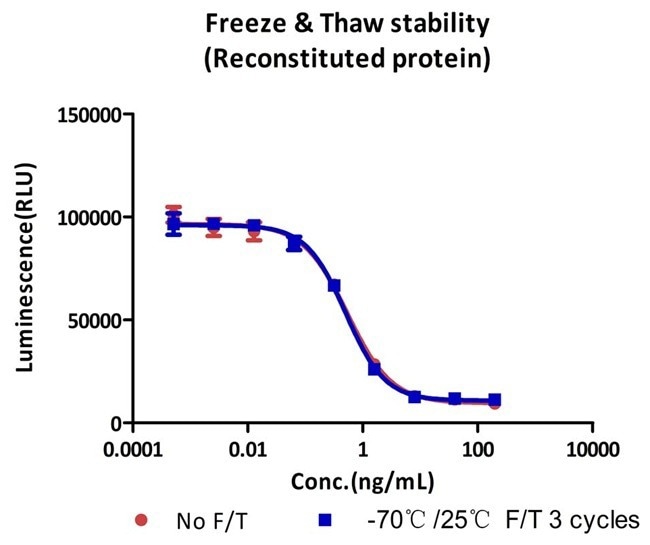
Image Credit: ACROBiosystems
Following three cycles of freezing and thawing, the cell-based assay demonstrates that GMP Human IFN-gamma Protein (Cat. No. GMP-IFGH24) remains stable.
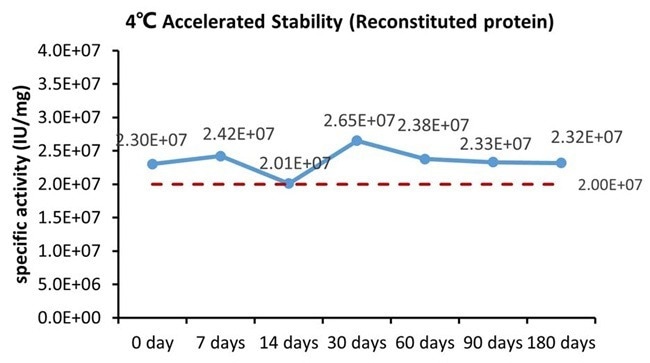
Image Credit: ACROBiosystems
GMP Human IFN-gamma Protein (Cat. No. GMP-IFGH24) is stable at 4 °C for six months, according to the cell-based assay.
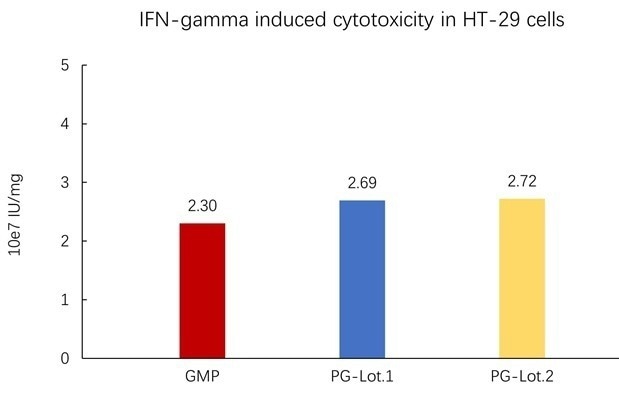
Image Credit: ACROBiosystems
Batch-to-batch consistency between PG IFN-gamma and Acro's GMP is demonstrated by the cell-based assay.