The serum-free culture medium CelTheraTM GMP T Cell Expansion Medium (Phenol Red-free) was created especially to support the culture of human T cells. It is a GMP-produced T cell maintenance and expansion medium that is free of serum and animal origin.
Animal origin-free culture media can enhance batch-to-batch consistency, prevent T cell overactivation by unknown serum components, and lower the risk of introducing potentially harmful microorganisms during the culture process more effectively than traditional culture media or xeno-free culture media.
In addition to maintaining the high fold expansion of T cells, CelTheraTM GMP T Cell Expansion Medium (Phenol Red-free) does not require the addition of any serum or serum replacements. If users decide to add serum or serum replacement, specific T cell applications should dictate the dosage.
Source: ACROBiosystems
| Cat. No. |
Components |
Size |
| GMP-CM3102 |
CelThera™ GMP T Cell Expansion Medium (Phenol Red-free) |
1000 mL |
| GMP-CM3101-1 |
CelThera™ GMP T Cell Expansion Supplement |
7.25 mL |
Product show

Image Credit: ACROBiosystems
Features
- Serum-free, animal origin-free (AOF), and free from exogenous growth factors.
- Designed to support low-density seeding and high fold expansion of T cells.
- Suitable for large-scale T cell expansion.
- No additional serum or serum replacements needed.
- T cell phenotypes comparable to media supplemented with serum or serum replacement.
- Contains only recombinant proteins, with no antibiotics in the formulation.
- Produced according to current GMP guidelines.
Storage
When kept between 2 °C and 8 °C and shielded from light, the CelTheraTM GMP T Cell Expansion Medium (Phenol Red-free) remains stable for 18 months.
When kept at -20 °C or lower and shielded from light, the CelTheraTM GMP T Cell Expansion Supplement remains stable for a full year.
ACRO quality management system
- QMS(ISO, GMP)
- Quality Advantages
- Quality Control Process
Application data
T cell viability and growth rate in different media
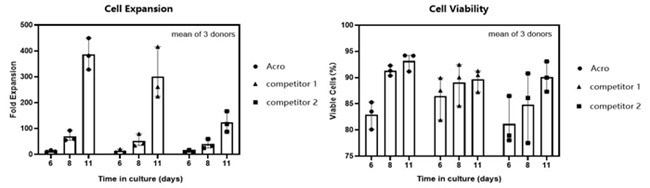
Image Credit: ACROBiosystems
T cells from PBMCs of three different donors were activated and cultured for 11 days in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Cell count and viability were assessed on days 6, 8, and 11 using trypan blue staining. Results showed that cells cultured in Acro T cell medium (Cat. No. GMP-CM3102) exhibited a faster proliferation rate and higher viability compared to the other two media.
Tcm ratio across different media
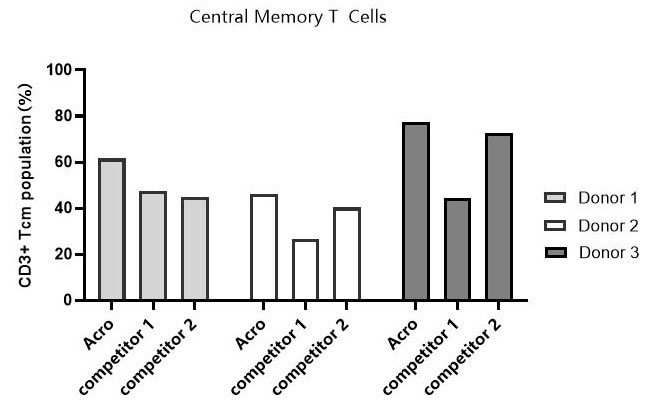
Image Credit: ACROBiosystems
T cells from PBMCs of three different donors were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). When the cells reached approximately 50-fold expansion, the percentage of central memory T cells (CD45RO+/CCR7+) was assessed by flow cytometry. Results showed that cells cultured in Acro T cell medium (Cat. No. GMP-CM3102) had a higher central memory T cell percentage compared to the other two media.
Efficiency of lentivirus transduction in different media
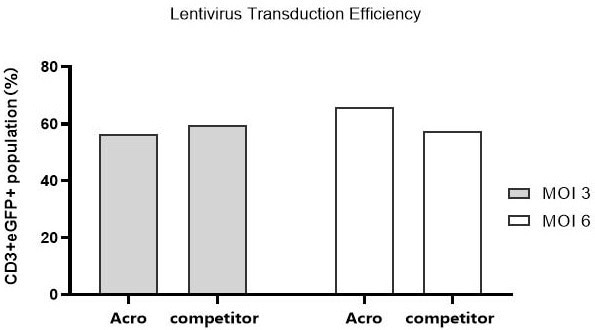
Image Credit: ACROBiosystems
T cells from PBMCs were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Twenty-four hours post-activation, the cells were transduced with pLenti-CMV-EGFP-puro lentivirus at an MOI of 3 or 6.
After another 24 hours, the lentivirus was removed by centrifugation, and the cells were cultured for an additional 48 hours. Flow cytometry analysis of the CD3+eGFP+ population showed that cells in Acro T cell medium (Cat. No. GMP-CM3102) had a lentiviral transduction efficiency comparable to that of the other medium.
Multiple donor verification
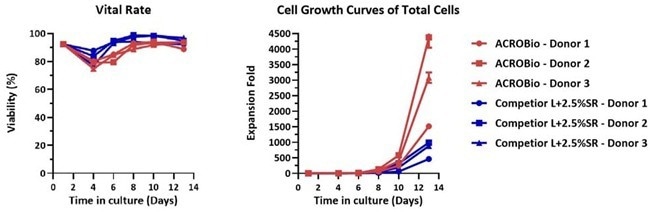
Image Credit: ACROBiosystems
Human PBMCs were cultured for two weeks with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) in either CelTheraTM GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3102) or T cell culture medium (Competitor L + 2.5% SR).
Results indicated that CelTheraTM GMP T Cell Expansion Medium performed comparably to Competitor L + 2.5 % SR, with notably better cell expansion observed in CelTheraTM GMP T Cell Expansion Medium (Cat. No. GMP-CM3102).
Large-scale culture verification
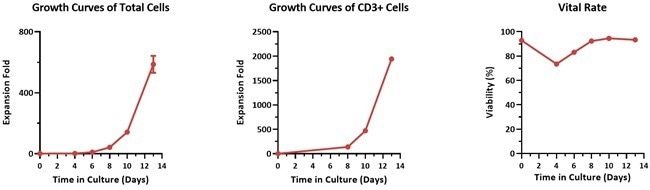
Image Credit: ACROBiosystems
Human PBMCs were activated using 0.2 µg/mL GMP Monoclonal Anti-Human CD3 Antibody (OKT3) (ACROBiosystems, Cat. No. GMP-MC0323) and 1 µg/mL GMP Monoclonal Anti-Human CD28 Antibody (ACROBiosystems, Cat. No. GMP-MC2824), then cultured for two weeks in CelTheraTM GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3102) supplemented with 500 IU/mL GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14).
Results demonstrated that this combination of GMP human IL-2 protein, GMP monoclonal anti-human CD3 and CD28 antibodies, and CelTheraTM GMP T Cell Expansion Medium effectively supports T cell culture in a 3L large-scale system, enabling efficient expansion with high viability.