Interleukin-2 (IL-2) is a cytokine, a type of immune system signaling molecule, that plays a crucial role in the body's response to microbial infections and in distinguishing between self and non-self. It exerts its effects by binding to IL-2 receptors, which are expressed on lymphocytes - the cells responsible for immunity.
Mature human IL-2 shares 56% and 66% amino acid sequence identity with mouse and rat IL-2, respectively, and exhibits cross-species activity with mouse IL-2. Its receptor consists of three subunits that exist in various preformed complexes on the cell surface.
IL-2 is also essential for T cell development in the thymus, particularly for the maturation of regulatory T cells (T-regs). Once these cells leave the thymus, they help prevent other T cells from mistakenly targeting self-antigens, which could lead to autoimmunity. T-regs achieve this by inhibiting the production of IL-2 in responding cells. This regulatory function underscores IL-2's critical role in maintaining immune balance and preventing autoimmune reactions.
Features
- Developed in compliance with ISO 9001:2015 and ISO 13485:2016 standards
- Produced and quality control (QC) tested in a GMP-compliant facility
- FDA DMF registered
- Composed of animal-free materials
- Free from beta-lactam components
- Ensures batch-to-batch consistency
- Undergoes rigorous quality control testing
Source
The GMP Human IL-2 Protein (GMP-L02H14) is derived from E. coli cells and includes the amino acids Ala 21 to Thr 153 (Accession # P60568-1).
Predicted N-terminus: Met
Molecular characterization

Image Credit: ACROBiosystems Inc
This protein is untagged. The calculated molecular weight is 15.4 kDa. When analyzed under reducing conditions (SDS-PAGE), it migrates as 16 kDa ± 2 kDa, based on calibration with the Star Ribbon Pre-stained Protein Marker.
Endotoxin
Contains less than 5.0 EU/vial, as determined by the LAL method.
Host cell protein
Contains less than 0.5 ng/µg of protein, as tested by ELISA.
Host cell DNA
Contains less than 0.1 ng/μg of protein, as tested by qPCR.
Sterility
Sterility testing was conducted using the membrane filtration method, following CP<1101>, USP<71>, and Eur. Ph. 2.6.1 guidelines.
Mycoplasma
Tested negative for mycoplasma contamination.
Purity
Purity exceeds 95%, as determined by SDS-PAGE analysis.
Formulation
Lyophilized from a 0.22 μm filtered solution containing phosphate and protectants.
Shipping
Supplied and shipped with blue ice.
Storage
Upon receipt, store immediately at -20 °C or lower for long-term preservation
Avoid repeated freeze-thaw cycles.
Stability:
At -20 °C: Stable for 5 years in lyophilized form.
At -70 °C: Stable for 12 months under sterile conditions after reconstitution.
ACRO quality management system
- QMS (ISO, GMP)
- Quality Advantages
- Comprehensive quality control processes ensure product reliability.
QC and assay protocol
SDS-PAGE
GMP Human IL-2 Protein in reducing (R) conditions on SDS-PAGE. Coomassie Blue was used to stain the gel. According to the Star Ribbon Pre-stained Protein Marker, the protein's purity is higher than 95%.
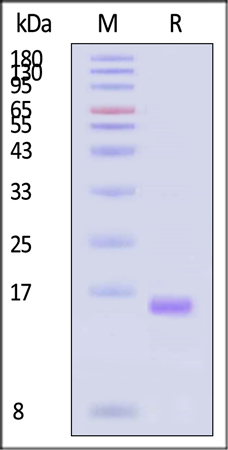
Image Credit: ACROBiosystems Inc
Bioactivity-Bioactivity CELL BASE
CTLL-2 cell proliferation is stimulated by GMP Human IL-2 Protein (Cat. No. GMP-L02H14). When compared to the human Interleukin-2 China National Standard (NIFDC code: 270008), the specific activity of GMP Human IL-2 Protein is ≥ 1.20×107 IU/mg (QC tested).
The human IL-2 WHO International Standard (NIBSC code: 86/500) was used to prepare and calibrate the China National Institutes for Food and Drug Control (NIFDC) Standard.
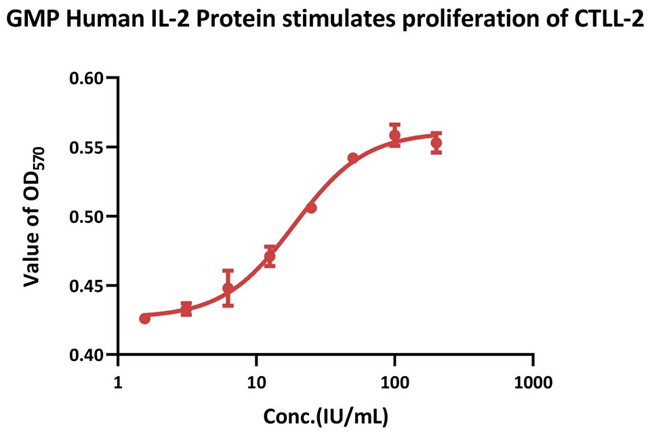
Image Credit: ACROBiosystems Inc
Application data
3 × 106 human PBMCs were cultivated with different concentrations of IL-2 Protein (Competitor N) or GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) for a week.
The CD3+ cell number was 4.8 × 105 and the CD3+ ratio was 47.6% for the negative control group (without IL-2). The outcome demonstrates that the activity of IL-2 Protein (Competitor N) and GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) is comparable.
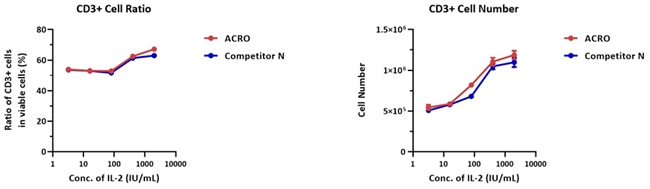
Image Credit: ACROBiosystems Inc
3 × 106 human PBMCs were cultivated with different concentrations of IL-2 Protein (Competitor N) or GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) for a week. The CD3+ CD4-CD8+ cell number was 1.0 × 105, and the CD3+ CD4-CD8+ ratio was 21.7% for the negative control group (without IL-2).
The outcome demonstrates that the activity of IL-2 Protein (Competitor N) and GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) is comparable.
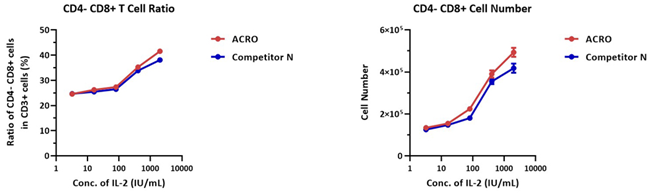
Image Credit: ACROBiosystems Inc
3 × 106 human PBMCs were cultivated with different concentrations of either IL-2 Protein (Competitor N) or GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) for seven days. The CD3+ CD4+ CD8-cell number was 3.5 × 105, and the CD3+ CD4+ CD8-ratio was 72.4% for the negative control group (without IL-2).
The outcome demonstrates that the activity of IL-2 Protein (Competitor N) and GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) is comparable.
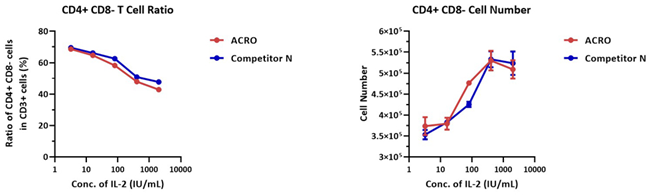
Image Credit: ACROBiosystems Inc
Bioactivity-Stability
According to the cell-based assay, GMP Human IL-2 Protein (Cat. No. GMP-L02H14) remains stable for eight hours at 37 °C.
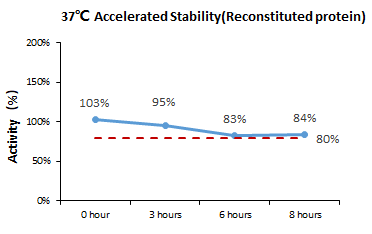
Image Credit: ACROBiosystems Inc
The GMP Human IL-2 Protein (Cat. No. GMP-L02H14) is stable across batches, according to the cell-based assay.
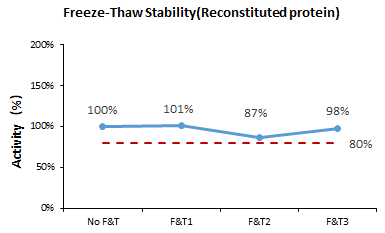
Image Credit: ACROBiosystems Inc
Following three cycles of freezing and thawing, the cell-based assay demonstrates that GMP Human IL-2 (Cat. No. GMP-L02H14) remains stable.
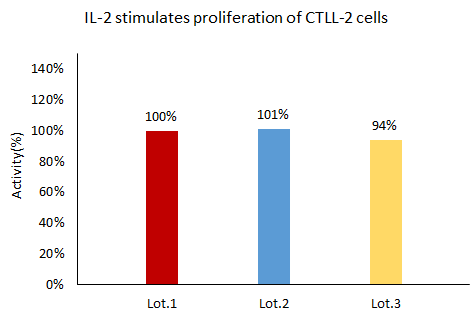
Image Credit: ACROBiosystems Inc