Interleukin-21 (IL-21) is a secreted protein that belongs to the IL-15/IL-21 cytokine family. It binds to a unique receptor, IL-21R, which partners with the common cytokine receptor gamma chain (γc). IL-21 influences various immune cells, including CD8+ memory T cells, NK cells, and specific subsets of CD4+ memory T cells.
IL-21R is widely expressed on lympho-hematopoietic cells, highlighting its broad role in immune regulation. Produced by CD4+ T cells in response to antigenic stimulation, IL-21 generally enhances antigen-specific immune responses. It also boosts the anti-tumor activity of CD8+ T cells and NK cells.
The IL-21/IL-21R interaction activates a signaling cascade involving the tyrosine kinases JAK1 and JAK3, stimulating the transcription factors STAT1 and STAT3. Notably, the γc component of IL-21R is also shared by other cytokine receptors, including those for IL-2, IL-4, IL-7, IL-9, and IL-15, underscoring IL-21’s role within a broader immune signaling network.
Features
- Developed in compliance with ISO 9001:2015 and ISO 13485:2016 standards
- Produced and quality control (QC) tested in a GMP-compliant facility
- FDA DMF registered
- Animal-free materials
- Free from beta-lactam components
- Ensures batch-to-batch consistency
- Undergoes rigorous quality control testing
Source
GMP Human IL-21 Protein (GMP-L21H25) is derived from human 293 cells (HEK293). It includes amino acids Gln 30 - Ser 162 (Accession # Q9HBE4-1).
- Predicted N-terminus: Gln 30
Molecular characterization

Image Credit: ACROBiosystems Inc
This protein is tag-free.
The calculated molecular weight is 15.5 kDa. Due to glycosylation, under reducing conditions (SDS-PAGE), the protein migrates as 16 kDa ± 3 kDa.
Endotoxin
Contains less than 10 EU/mg as determined by the LAL method.
Host cell protein
Less than 0.5 ng/µg of protein, as tested by ELISA.
Host cell DNA
Less than 0.02 ng/μg of protein, as determined by qPCR.
Sterility
Sterility testing was conducted using the membrane filtration method, following CP<1101>, USP<71>, and Eur. Ph. 2.6.1 guidelines.
Mycoplasma
Tested negative.
Purity
Greater than 95% purity, as confirmed by SDS-PAGE.
Formulation
Lyophilized from a 0.22 μm filtered solution in PBS, pH 7.4, with added protectants.
Shipping
Supplied and shipped with blue ice.
Storage
Upon receipt, store immediately at -20 °C or lower for long-term preservation.
Avoid repeated freeze-thaw cycles.
Stability:
- Lyophilized state: Stable for 5 years at -20 °C to -70 °C.
- After reconstitution under sterile conditions: Stable for 12 months at -70 °C.
ACRO quality management system
- QMS (ISO, GMP)
- Quality Advantages
- Quality Control Process
QC and assay protocol
SDS-PAGE
GMP Human IL-21 Protein on SDS-PAGE under reducing (R) condition. Coomassie Blue was used to stain the gel. The protein has a purity of over 95%.
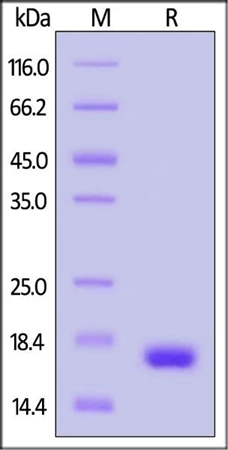
Image Credit: ACROBiosystems Inc
Bioactivity-Bioactivity CELL BASE
NK-92 human natural killer lymphoma cells stimulated with 10 ng/mL GMP Human IL-15 Protein (Cat. No. GMP-L15H13) secrete more IFN-γ when stimulated with GMP Human IL-21 Protein (Cat. No. GMP-L21H25). According to a QC test, the specific activity of GMP Human IL-21 Protein (Cat. No. GMP-L21H25) is greater than 1.00 × 105 U/mg.
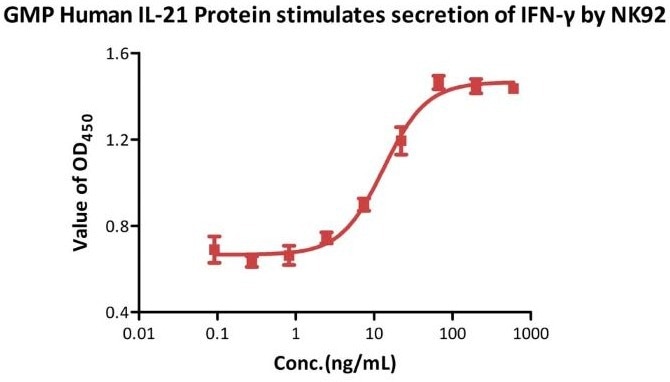
Image Credit: ACROBiosystems Inc
Bioactivity-Stability
The GMP Human IL-21 Protein (Cat. No. GMP-L21H25) is stable at 37 °C for 24 hours, according to the cell-based assay.
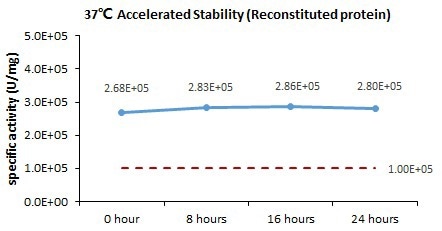
Image Credit: ACROBiosystems Inc
The cell-based assay shows that the GMP Human IL-21 Protein (Cat. No. GMP-L21H25) is stable following three cycles of freezing and thawing.
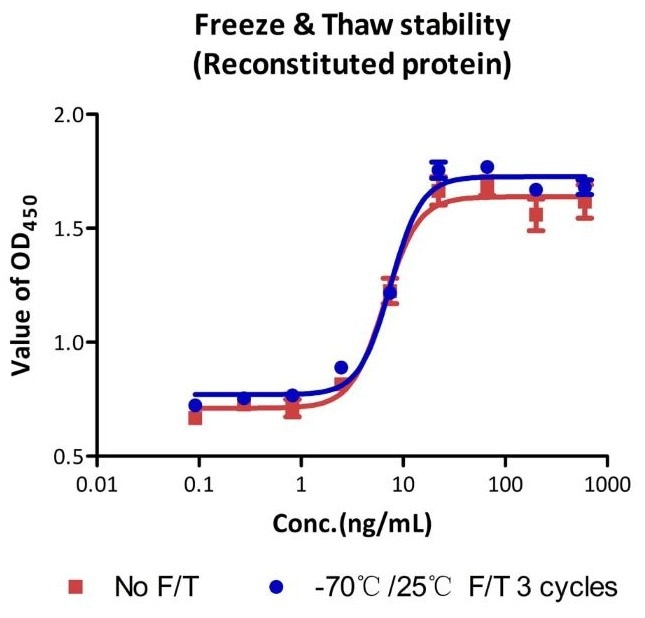
Image Credit: ACROBiosystems Inc
The GMP Human IL-21 Protein (Cat. No. GMP-L21H25) is stable at 4 °C for six months, according to the cell-based assay.
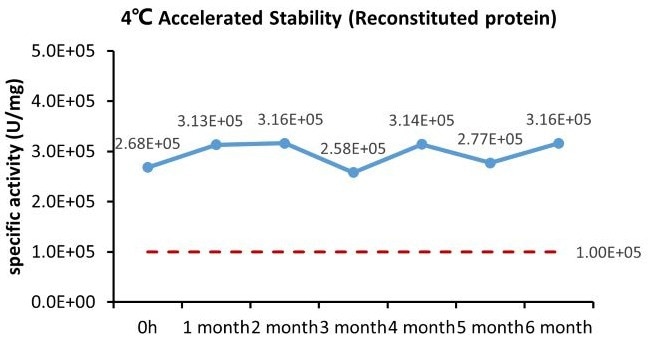
Image Credit: ACROBiosystems Inc
The cell-based assay demonstrates batch-to-batch consistency between Acro's GMP and PG IL-21.
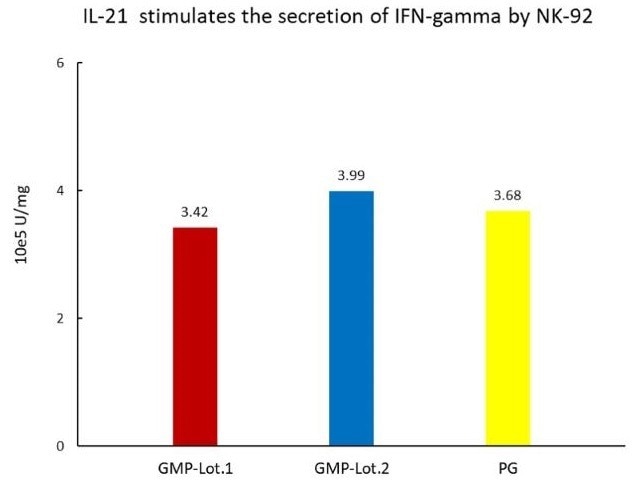
Image Credit: ACROBiosystems Inc