As the COVID-19 pandemic progresses with little prospect of immediate relief, a recent study by Johns Hopkins University researchers published on the preprint server medRxiv* in October 2020 discusses the association of Immunoglobulin M (IgM) autoantibodies against the host receptor that binds severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with severe disease via vascular endothelial damage. This could help develop better therapies and prognostic criteria.
Statistically, up to 10% of patients with COVID-19 are hospitalized, and about 5% are admitted to intensive care units (ICUs). In the USA, mortality rates of hospitalized patients range between 13 and 28%. Evidence suggests that severe and terminal disease is related to abnormal inflammation and activation of the infection's immune system.
This is reflected in acute respiratory distress syndrome (ARDS) occurrence in a sizable number of patients. In addition, many show signs of vascular damage, hypercoagulation, and complications related to heart function, but in which complement activation has been implicated.
The role of complement-mediated inflammation is supported by the finding that low doses of dexamethasone exert a beneficial effect on some COVID-19 patients with respiratory distress serious enough to require ventilatory assistance, reducing the mortality rate. Thus, it is essential to uncover pathways of dysregulated inflammation behind severe COVID-19 and mitigate them, if possible, by already readily available tools.
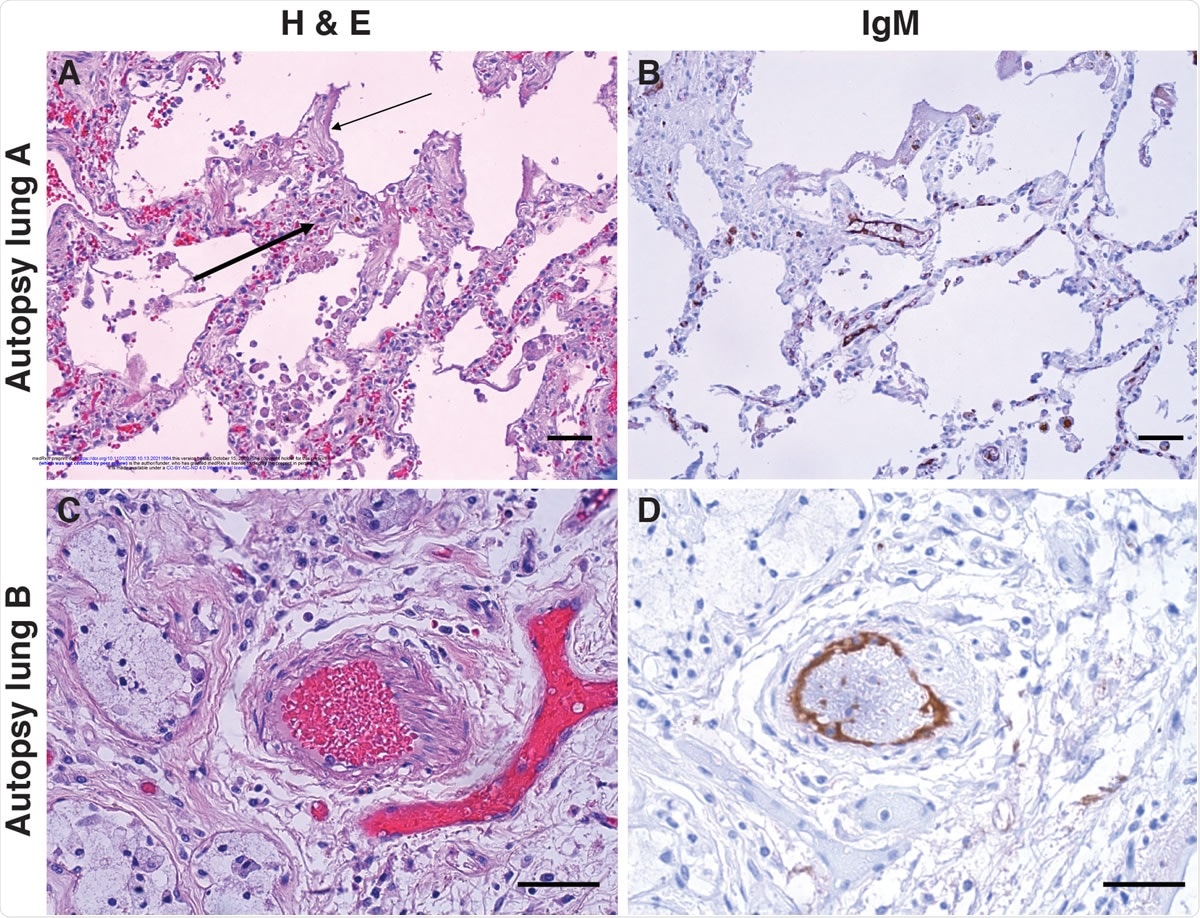
IgM deposition on endothelium in COVID-19 lung. Lung paraffin sections from two autopsy patients (lung A, upper panels; lung B, lower panels) were stained with hematoxylin and eosin (A & C) or with an anti-IgM antibody (B & D). A: A section of the left upper lobe of the lung shows a widened interstitium with capillaries showing reactive endothelium (thick arrow). There are hyaline membranes lining alveolar spaces (thin arrow), consistent with the exudative phase of diffuse alveolar damage (acute lung injury). B: Anti-IgM immunohistochemical staining of the same tissue highlights capillary endothelium in that area. C: A small artery of a bronchiole stained with hematoxylin and eosin, with (D) endothelial staining for anti-IgM. Size bars represent 50 microns.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Higher Frequency of anti-ACE2 IgM in Severe COVID-19
Some patients with severe COVID-19 have been found to have IgM autoantibodies, which attracted attention to the potential role of ACE2 as an autoantigen in this condition. The viral spike protein has 5-20 times higher affinity to the ACE2 receptor compared to other coronaviruses that also attach to this site, by higher.
This protein is also expressed more highly in the lung's epithelium and the endothelium of pulmonary vasculature and the heart. ACE2 mutations associated with reduced function are related to poor outcomes in ARDS. Therefore, the current study examined a group of 66 hospitalized patients with COVID-19 for autoantibodies to ACE2, either IgG or IgM.
All patients had moderate to severe COVID-19 (28 severe and 38 moderate). Among these, a quarter of the severe COVID-19 group (n=8) had IgM autoantibodies for ACE2, of which 7 had required mechanical ventilation or were deceased. These two correspond to WHO ordinal scales 6/7 and 8, respectively.
However, among the 38 'moderate' patients, only 1 had these autoantibodies or <3%. The risk for autoantibodies to ACE2 is thus 12-fold higher among severe COVID-19 patients.
The researchers then enlarged the sample size and duration of measurement, using residual serum from repeated blood draws taken from 52 hospitalized patients over their stay in hospital.
Of these additional patients, 38 had severe disease, 31 being ventilated and 7 deceased. The remaining 14 patients had a moderate disease. Here again, 'severe' patients had ~30% positivity for IgM against ACE2 while in the milder group, the incidence was 7%.
IgM ACE2 Antibody Frequency Seven-fold in Severe COVID-19
Overall, therefore, ~27% of 66 'severe' COVID-19 patients had autoantibodies, compared to ~4% of moderately sick patients, indicating a 9-fold increased risk of autoimmunity in the former. Again, 18% in the first and ~12% in the second category had IgG autoantibodies to ACE2.
The ACE2 receptor thus appears to be a primary target of autoantibodies in patients with COVID-19. IgM autoantibodies were strongly linked to severe disease.
Patients with IgM autoantibodies were ~62 years on average, compared to 59 years in the negative group, and 72% of them were female. However, there was no significant association of anti-ACE2 antibodies with sex. The BMI in patients with autoantibodies was ~35 compared to 30 in those without.
Clinical Indicators of Inflammation in IgM Positives
IgM-positives had significantly raised mean temperatures over the first 10 days in hospital compared to the IgM-negative group. CRP levels during this period were also raised, the peak and average values in the IgM-positives being 20mg/dL and ~17mg/dL vs. 7.4mg/dL and ~14 mg/dL for the IgM-negative group, respectively.
Anti-ACE2 antibodies were absent in patients with other infections and autoimmune disease except for a single patient with a rare dermatopulmonary syndrome. This autoimmune disease has several clinical features in common with severe COVID-19. The occurrence of common clinical features and IgM anti-ACE2 antibodies in both instances is interesting and warrants further study.
Longitudinal Analysis Of Anti-ACE2 IgM And IgG
In only three patients, testing covered the whole period before and after antibody detection. This showed the autoantibodies' first appearance at ~10 days from admission, about the time when the clinical phenotype worsened to the point when intubation was required.
At the first point of testing, IgM against ACE2 was already high in a majority of patients who had already been intubated. In 4 of these patients, the levels did not change, while in the rest, the autoantibody levels declined with time. This is consistent with class switching, typically seen in T-cell mediated cellular immunity, where IgM switches to IgG.
However, this was not supported by the failure to detect a rise in anti-ACE2 IgG levels over time. In one case, both IgG and IgM were initially detected and remained at the same level over time. On the other hand, in 8 other patients, IgM was found to fall by half during the testing period, while IgG was not detected.
T-cell Independent Antibody Response
Previous research has repeatedly demonstrated high admission levels of anti-spike IgG to be correlated with the severity of COVID-19. This was confirmed in this study. However, patients with IgM positivity also had three-fold higher anti-S IgG than those who were negative.
All patients with anti-ACE2 IgM antibodies were also positive for anti-ACE2 IgG, vs. half of those without the former. This cannot be attributed to a general failure of antiviral class switching from IgM to IgG since the IgG production is strongly found in these patients.
This militates against the IgM autoantibody production against ACE2 being T cell-dependent and suggests it arises from B cells instead. This would agree with the finding of plasmablast expansion in severe COVID-19.
ACE2 IgM Antibodies Marker for Inflammation and Recovery
The researchers suggest, "An intriguing possibility is that a robust neutralizing anti-S IgG response induces an anti-idiotype IgM response, which also cross-reacts with ACE2, the spike protein receptor." This could also explain the delay in the emergence of anti-ACE2 IgM and the higher inflammatory features in this subset of patients, suggesting the disease is intensifying in severity before clinical signs of failure appear.
Steroids were found to be useful in reducing the mortality risk in severely ill COVID-19 patients. The researchers found that none had received steroids for over two days among the deceased IgM-positive individuals in the current study. Among the 13 patients on ventilation who survived, six had received these drugs for over two days. There was a time-related reduction in anti-ACE2 IgM level correlated with steroid therapy in the three patients with longitudinal follow-up, as mentioned above.
This may indicate anti-ACE2 IgM's potential as a biomarker of severe disease and recovery following immunomodulatory therapies.
IgM ACE2 Autoantibodies Associated with Vasculopathy
The investigators found that the IgM antibodies have not undergone affinity maturation, thus having low affinity, in the high nanomolar to the micromolar range, but with greater avidity. However, they do not reduce the ACE2 function.
On the other hand, high-affinity IgM antibodies were strongly associated with complement activation following antigen binding. This suggests that they are key to the extensive complement activation seen in COVID-19.
Moreover, the lungs in COVID-19 patients show damage centered around the pulmonary vasculature, as well as severe endothelial membrane damage and ACE2 expression. In addition, capillary damage and occlusion are associated with angiogenesis. These features accompany the expected widespread alveolar injury and infiltrating lymphocytes around the blood vessels.
Endothelial IgM staining shows that IgM is bound to an endothelial target with the same distribution as ACE2 in severely ill COVID-19 patients. It remains to be urgently determined whether the target of this IgM is indeed ACE2 or another molecule.
Implications and Future Directions
The researchers postulate that the anti-ACE2 IgM is harmful to the patient via endothelial binding, which results in classical complement activation, triggering an inflammatory response. If true, this could shape therapeutic strategies such as short regimens of anti-inflammatories like steroids, or IVIG, complement inhibitors, and molecules that inhibit antibody production via T-independent pathways, instead of much more broad-based and intensive immunosuppression to inhibit T cell-mediated immunity.
The commonality of phenotype and autoantibody between COVID-19 and the anti-MDA5-associated dermatopulmonary syndrome may also extend to the beneficial effect of the above immunosuppressive drugs.
The researchers sum up: "This study adds a unique biomarker that results from SARS-CoV-2 infection and is strongly associated with severe clinical outcomes in patients with COVID-19."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources